|
~80% |
|
~53% |
|
~30% |
|
~77% |
|
~84% |
|
~81% |
|
~0% |
|
~% |
|
~59% |
|
~72% |
|
~57% |
|
~% |
|
~76% |
|
~% |
|
~% |
|
~67% |
|
~% |
|
~% |
|
~60% |
|
~% |
|
~73% |
|
~% |
|
~% |
|
~66% |
|
~93% |
|
~81% |
|
~90% |
|
~81% |
|
~% |
|
~% |
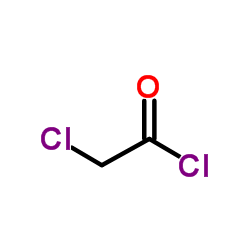

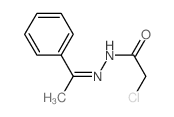
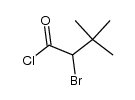
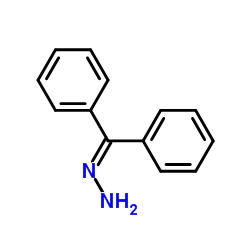
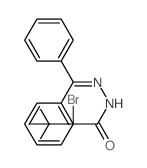

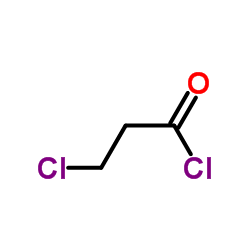

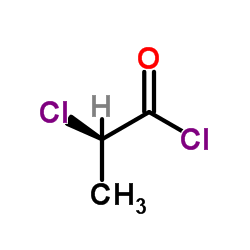
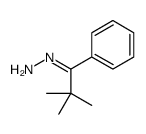
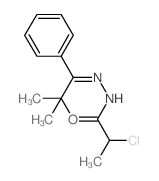

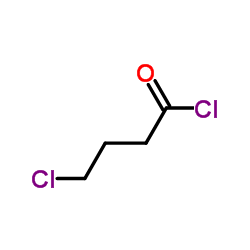
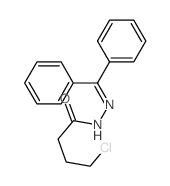

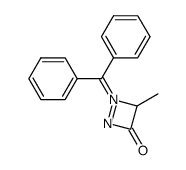
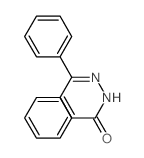

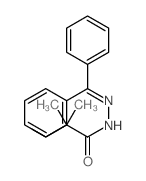
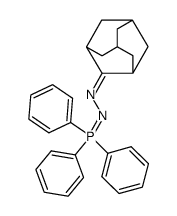
![Acetic acid, 2-chloro-,2-(tricyclo[3.3.1.13,7]dec-2-ylidene)hydrazide Structure](https://image.chemsrc.com/caspic/478/79289-19-3.png)
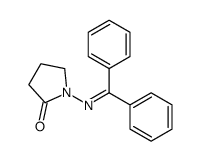

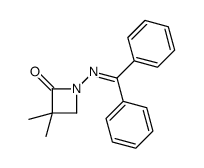

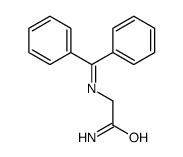

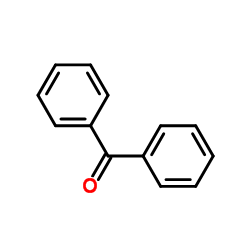
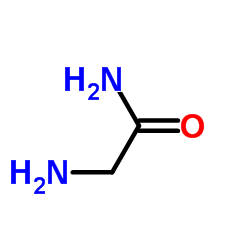

![3-chloro-N-[(2,2-dimethyl-1-phenyl-propylidene)amino]propanamide Structure](https://image.chemsrc.com/caspic/009/79289-23-9.png)

