lipoic acid
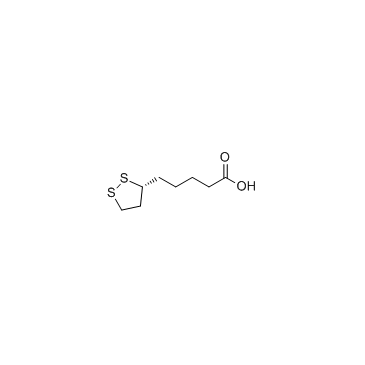
lipoic acid structure
|
Common Name | lipoic acid | ||
|---|---|---|---|---|
| CAS Number | 1200-22-2 | Molecular Weight | 206.326 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 362.5±11.0 °C at 760 mmHg | |
| Molecular Formula | C8H14O2S2 | Melting Point | 46-49ºC | |
| MSDS | Chinese USA | Flash Point | 173.0±19.3 °C | |
|
2-Styrylchromones: novel strong scavengers of reactive oxygen and nitrogen species.
Bioorg. Med. Chem. 15 , 6027-36, (2007) 2-Styrylchromones are a small group of naturally occurring chromones, vinylogues of flavones (2-phenylchromones). Natural and synthetic 2-styrylchromones have been tested in different biological systems, showing activities with potential therapeutic applicati... |
|
|
Synthesis and antioxidant properties of new chromone derivatives.
Bioorg. Med. Chem. 17 , 7218-26, (2009) Nowadays, the recognition of the benefits of antioxidants is eliciting an increasingly interest in the search for new molecules with improved activity. The aim of the present work was to search for improved reactive oxygen species (ROS) and reactive nitrogen ... |
|
|
Synthesis and biological evaluation of 3,7-diazabicyclo[4.3.0]nonan-8-ones as potential nootropic and analgesic drugs.
J. Med. Chem. 54 , 2512-6, (2011) A series of cis and trans 3,7-diazabicyclo[4.3.0]nonan-8-ones has been synthesized and tested for their ability to revert scopolamine-induced amnesia in the mouse passive-avoidance test. The racemates of the most potent compounds 4 and 7 were separated and te... |
|
|
Diaryl-dithiolanes and -isothiazoles: COX-1/COX-2 and 5-LOX-inhibitory, *OH scavenging and anti-adhesive activities.
Bioorg. Med. Chem. 17 , 558-68, (2009) Three series of non-steroidal anti-inflammatory drugs (NSAIDs) inhibiting the cyclooxygenase/5-lipoxygenase (COX/5-LOX) pathways as such as formation of hydroxyl radicals and adhesion were prepared: 4,5-diaryl isothiazoles, 4,5-diaryl 3H-1,2-dithiole-3-thione... |
|
|
Investigations concerning the COX/5-LOX inhibiting and hydroxyl radical scavenging potencies of novel 4,5-diaryl isoselenazoles.
Eur. J. Med. Chem. 43 , 1152-9, (2008) The aim of this study was to investigate 4,5-diaryl isoselenazoles as multiple target non-steroidal anti-inflammatory drugs (MTNSAIDs) which can intervene into the inflammatory processes via different mechanisms of action creating a new class of compounds. He... |
|
|
Design and synthesis of novel quinolinone-3-aminoamides and their alpha-lipoic acid adducts as antioxidant and anti-inflammatory agents.
J. Med. Chem. 50 , 2450-8, (2007) A series of N-substituted-quinolinone-3-aminoamides and their hybrids containing the alpha-lipoic acid functionality were designed and synthesized as potential bifunctional agents combining antioxidant and anti-inflammatory activity. The new compounds were ev... |
|
|
Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts.
Eur. J. Med. Chem. 44 , 3020-6, (2009) In the present work a series of novel coumarin-3-carboxamides and their hybrids with the alpha-lipoic acid were designed, synthesized and tested as potent antioxidant and anti-inflammatory agents. The new compounds were evaluated for their antioxidant activit... |
|
|
Pharmacokinetics of alpha-lipoic acid in subjects with severe kidney damage and end-stage renal disease.
J. Clin. Pharmacol. 45(3) , 313-28, (2005) In an open-label, parallel-group study involving 16 patients (8 with severely reduced renal function, 8 with end-stage renal disease needing hemodialysis), the effect of renal function on the pharmacokinetics, metabolism, and safety and of alpha-lipoic acid (... |
|
|
Lipoic (thioctic) acid increases brain energy availability and skeletal muscle performance as shown by in vivo 31P-MRS in a patient with mitochondrial cytopathy.
J. Neurol. 242(7) , 472-7, (1995) A woman affected by chronic progressive external ophthalmoplegia and muscle mitochondrial DNA deletion was studied by phosphorus magnetic resonance spectroscopy (31P-MRS) prior to and after 1 and 7 months of treatment with oral lipoic acid. Before treatment a... |
|
|
Fluorinated amphiphilic amino acid derivatives as antioxidant carriers: a new class of protective agents.
J. Med. Chem. 49 , 2812-20, (2006) The use of classical antioxidants is limited by their low bioavailabilities, and therefore, high doses are usually required to display significant protective activity. In a recent article (J. Med. Chem. 2003, 46, 5230) we showed that the ability of the alpha-... |