Fmoc-Asp-OH
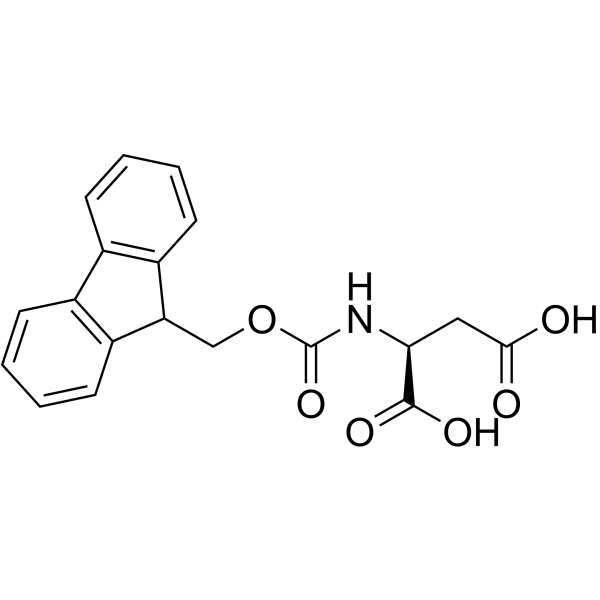
Fmoc-Asp-OH structure
|
Common Name | Fmoc-Asp-OH | ||
|---|---|---|---|---|
| CAS Number | 119062-05-4 | Molecular Weight | 355.341 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 587.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H17NO6 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 308.9±28.7 °C | |
|
Synthesis of N-linked glycopeptides via solid-phase aspartylation.
Org. Biomol. Chem. 8 , 3723-3733, (2010) An efficient strategy for the preparation of N-linked glycopeptides is described. The method relies on the use of side chain protecting groups on aspartic acid residues, namely the allyl and Dmab esters, which are orthogonal to those utilised in Fmoc-strategy... |
|
|
The aspartimide problem in Fmoc-based SPPS. Part I.
J. Pept. Sci. 9 , 36-46, (2003) A variety of Asp beta-carboxy protecting groups, Hmb backbone protection and a range of Fmoc cleavage protocols have been employed in syntheses of the model hexapeptide H-VKDGYI-OH to investigate the aspartimide problem in more detail. The extent of formation... |
|
|
The aspartimide problem in Fmoc-based SPPS. Part II.
J. Pept. Sci. 9 , 518-526, (2003) The sequence dependence of base-catalysed aspartmide formation during Fmoc-based SPPS was systematically studied employing the peptide models H-Val-Lys-Asp-Xaa-Tyr-Ile-OH. The extent of formation of aspartimide and related by-products was determined by RP-HPL... |
|
|
The aspartimide problem in Fmoc-based SPPS. Part III.
J. Pept. Sci. 11 , 650-657, (2005) A newly developed Fmoc-Asp derivative, Fmoc-Asp beta-(2,3,4-trimethyl-pent-3-yl) ester, has been tried in the Fmoc-based SPPS of H-Val-Lys-Asp-Xaa-Tyr-Ile-OH, a well-established peptide model for studying base-catalysed aspartimide formation. When synthesizin... |
|
|
Synthesis and CD structural studies of CD52 peptides and glycopeptides.
Carbohydr. Res. 343 , 2894-2902, (2008) The syntheses of five natural and N-terminal acetylated peptides and glycopeptides of the CD52 antigen are described. Solid phase peptide synthesis was employed in the construction of the target compounds from Fmoc-protected commercial amino acids and synthet... |
|
|
On-resin convergent synthesis of a glycopeptide from HIV gp120 containing a high mannose type N-linked oligosaccharide.
Methods Mol. Biol. 751 , 343-355, (2011) This chapter describes a rapid and efficient approach for the solid-phase synthesis of N-linked glycopeptides that utilizes on-resin glycosylamine coupling to produce N-linked glycosylation sites. In this method, the full-length nonglycosylated peptide is fir... |