Picene
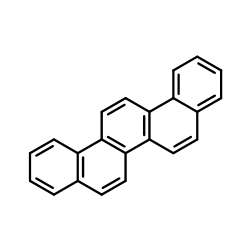
Picene structure
|
Common Name | Picene | ||
|---|---|---|---|---|
| CAS Number | 213-46-7 | Molecular Weight | 278.347 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 519.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C22H14 | Melting Point | 366-367° | |
| MSDS | Chinese USA | Flash Point | 264.5±15.1 °C | |
|
Comparative carcinogenicity of picene and dibenz[a,h]anthracene in the rat.
Biochem. Biophys. Res. Commun. 290(1) , 275-9, (2002) Early carcinogenicity tests found no evidence of activity for picene but found considerable initiating and carcinogenic activity for dibenz[a,h]anthracene (DBA). More recent investigation suggested that both pentacyclics were complete carcinogens when adminis... |
|
|
Tracer-based source apportionment of polycyclic aromatic hydrocarbons in PM2.5 in Guangzhou, southern China, using positive matrix factorization (PMF).
Environ. Sci. Pollut. Res. Int. 20(4) , 2398-409, (2013) From 28 November to 23 December 2009, 24-h PM2.5 samples were collected simultaneously at six sites in Guangzhou. Concentrations of 18 polycyclic aromatic hydrocarbons (PAHs) together with certain molecular tracers for vehicular emissions (i.e., hopanes and e... |
|
|
Phonon dynamics and electron-phonon coupling in pristine picene.
Phys. Chem. Chem. Phys. 14(5) , 1694-9, (2012) The paper reports a complete analysis of the phonon structure of crystalline picene, a recently announced organic semiconductor. Both lattice and intramolecular vibrations are investigated. An exhaustive assignment of lattice phonons is obtained through polar... |
|
|
New synthetic approaches to polycyclic aromatic hydrocarbons and their carcinogenic oxidized metabolites: derivatives of benzo[s]picene, benzo[rst]pentaphene, and dibenzo[b,def]chrysene.
J. Org. Chem. 65(13) , 3952-60, (2000) A new synthetic approach to polycyclic aromatic compounds is described that entails in the key steps double Suzuki coupling of PAH bisboronic acid derivatives with o-bromoaryl aldehydes to furnish aryl dialdehydes that are converted to larger polycyclic aroma... |
|
|
Organic superconductors.
Chem. Rec. 11(3) , 124-45, (2011) The present status of organic superconductors of charge-transfer (CT) type based on donor molecules is reviewed. Along with the superconducting phases of such materials and also of oxide superconductors, reside spin-ordered phases such as spin-density wave (S... |
|
|
Sensitivity and bias of molecular marker-based aerosol source apportionment models to small conltibutions of coal combustion soot.
Environ. Sci. Technol. 43(20) , 7770-7, (2009) Carbonaceous atmospheric particulate matter (PM25) collected in the midwestern United States revealed that soot emissions from incomplete coal combustion were important sources of several organic molecular markers used in source apportionment studies. Despite... |
|
|
Comparative tumorigenicity of picene and dibenz[a,h]anthracene in the mouse.
Carcinogenesis 11(10) , 1721-6, (1990) The carcinogenic activity of the two polycyclic aromatic hydrocarbons (PAHs), picene (benzo[a]chrysene) and dibenz[a,h]anthracene (DBA), was determined in NMRI mice by five different experimental protocols in order to find out if picene is a carcinogen as pre... |
|
|
Microsomal metabolism of picene.
Chem. Biol. Interact. 66(3-4) , 157-75, (1988) Picene, a polycyclic aromatic hydrocarbon (PAH) of environmental relevance has recently been predicted to be carcinogenic, based on quantum mechanical calculation, although in several animal studies no carcinogenicity could be detected. In order to find out i... |