2-Hexadecanone
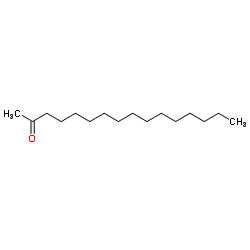
2-Hexadecanone structure
|
Common Name | 2-Hexadecanone | ||
|---|---|---|---|---|
| CAS Number | 18787-63-8 | Molecular Weight | 240.425 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 318.4±0.0 °C at 760 mmHg | |
| Molecular Formula | C16H32O | Melting Point | 43-45ºC(lit.) | |
| MSDS | USA | Flash Point | 82.7±13.2 °C | |
|
Identification of the female-produced sex pheromone of the scarab beetle, Hoplia equina.
J. Chem. Ecol. 29(7) , 1635-42, (2003) Hoplia equina LeConte (Coleoptera Scarabaeidae: Melolonthinae) is a beetle pest of cranberry beds in Massachusetts. Larvae feed on the roots of the cranberry plant, reducing yield as well as vine density. The female sex pheromone was identified as 2-tetradeca... |
|
|
A glucose/hydrogen peroxide biofuel cell that uses oxidase and peroxidase as catalysts by composite bulk-modified bioelectrodes based on a solid binding matrix.
Bioelectrochemistry 56(1-2) , 99-105, (2002) An improved composite bulk-modified bioelectrode setup based on a solid binding matrix (SBM) has been used to develop a glucose/hydrogen peroxide biofuel cell. Fuel is combined through a catalytically promoted reaction with oxygen into and oxidized species an... |
|
|
Cycloalkanones. 9. Comparison of analogues which inhibit cholesterol and fatty acid synthesis.
J. Med. Chem. 19(10) , 1257-61, (1976) A number of 2,8-dibenzylcyclooctanone analogues inhibited the HMG-CoA reductases activity of Holtzman male rat liver, whereas only 2-octanone, 2-hexadecanone, 2,8-dibenzylcyclooctanone derivatives, and 2-bis(4-chlorophenyl)-3,5-dimethyltetrahydro-4-pyrone inh... |
|
|
Cycloalkanones. 8. Hypocholesterolemic activity of long-chain ketones related to pentadecanone.
J. Med. Chem. 19(2) , 219-22, (1976) Aliphatic analogs of 2,8-dibenzylcyclooctanone which includes C15-C18 ketones have been investigated for hypocholesterolemic activity in rats. The position of the carbonyl group in the chain for maximum activity appears to be the 2 position. 2-Hexadecanone re... |
|
|
Lipids in the femoral gland secretions of male Schreiber's green lizards, Lacerta schreiberi.
Z. Naturforsch., C, J. Biosci. 61(9-10) , 763-8, (2006) In spite of the importance of chemoreception and chemical signals in social organization of lizards, only a few studies have examined the chemical composition of secretions that lizards use for intraspecific communication. The secretion of the femoral glands ... |
|
|
Amperometric pH-sensing biosensors for urea, penicillin, and oxalacetate. Stred'anský M, et al.
Anal. Chim. Acta 415(1) , 151-157, (2000)
|