5-Methyluridine
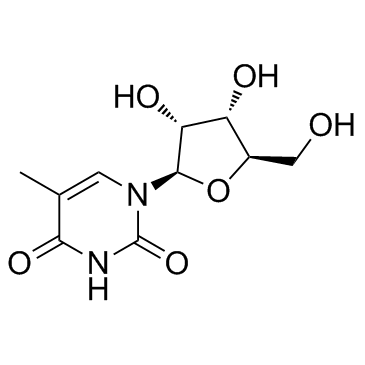
5-Methyluridine structure
|
Common Name | 5-Methyluridine | ||
|---|---|---|---|---|
| CAS Number | 1463-10-1 | Molecular Weight | 258.228 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C10H14N2O6 | Melting Point | 183-184 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
|
Metabolome analysis via comprehensive two-dimensional liquid chromatography: identification of modified nucleosides from RNA metabolism.
Anal. Bioanal. Chem 407(13) , 3555-66, (2015) Modified nucleosides derived from the RNA metabolism constitute an important chemical class, which are discussed as potential biomarkers in the detection of mammalian breast cancer. Not only the variability of modifications, but also the complexity of biologi... |
|
|
Exometabolom analysis of breast cancer cell lines: Metabolic signature.
Sci. Rep. 5 , 13374, (2015) Cancer cells show characteristic effects on cellular turnover and DNA/RNA modifications leading to elevated levels of excreted modified nucleosides. We investigated the molecular signature of different subtypes of breast cancer cell lines and the breast epith... |
|
|
Least absolute shrinkage and selection operator and dimensionality reduction techniques in quantitative structure retention relationship modeling of retention in hydrophilic interaction liquid chromatography.
J. Chromatogr. A. 1403 , 54-62, (2015) The objective of this study was to model the retention of nucleosides and pterins in hydrophilic interaction liquid chromatography (HILIC) via QSRR-based approach. Two home-made (Amino-P-C18, Amino-P-C10) and one commercial (IAM.PC.DD2) HILIC stationary phase... |
|
|
Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity.
PLoS Genet. 11 , e1005586, (2015) tRNA is the most highly modified class of RNA species, and modifications are found in tRNAs from all organisms that have been examined. Despite their vastly different chemical structures and their presence in different tRNAs, occurring in different locations ... |
|
|
Kilo-scale synthesis process for 2'-O-(2-methoxyethyl)-pyrimidine derivatives.
Nucleosides Nucleotides Nucleic Acids 24(5-7) , 815-8, (2005) We describe an improved process to produce 2'-O-(2-methoxyethyl)-pyrimidines. Starting with commercially available O-2,2'-anhydro-5-methyluridine and tris-(2-methoxyethyl)borate, we modified the ring-opening reaction conditions and changed to a continuous ext... |
|
|
Improved synthesis of 2'-deoxyadenosine and 5-methyluridine by Escherichia coli using an auto-induction system.
World J. Microbiol. Biotechnol. 28(2) , 721-7, (2012) Nucleoside analogues are used widely for the treatment of viral diseases and cancer, however the preparation of some important intermediates of these nucleoside analogues, including 2'-deoxyadenosine (dAR) and 5-methyluridine (5-MU), remains inconvenient. To ... |
|
|
The mere lack of rT modification in initiator tRNA does not facilitate formylation-independent initiation in Escherichia coli.
J. Bacteriol. 183(24) , 7397-402, (2001) Formylation of initiator methionyl-tRNA is essential for normal growth of eubacteria. However, under special conditions, it has been possible to initiate protein synthesis with unformylated initiator tRNA even in eubacteria. Earlier studies suggested that the... |
|
|
Evaluation of molecularly imprinted polymers using 2',3',5'-tri-O-acyluridines as templates for pyrimidine nucleoside recognition.
Anal. Bioanal. Chem 406(25) , 6275-84, (2014) In this paper, we describe the synthesis and evaluation of molecularly imprinted polymers (MIPs), prepared using 2',3',5'-tri-O-acyluridines as 'dummy' templates, for the selective recognition of uridine nucleosides. The MIPs were synthesised using a non-cova... |
|
|
Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA.
J. Biol. Chem. 281(4) , 2104-13, (2006) 2-Thioribothymidine (s(2)T) is a modified nucleoside of U, specifically found at position 54 of tRNAs from extreme thermophilic microorganisms. The function of the 2-thiocarbonyl group of s(2)T54 is thermostabilization of the three-dimensional structure of tR... |
|
|
Molecularly imprinted polymer of 5-methyluridine for solid-phase extraction of pyrimidine nucleoside cancer markers in urine.
Bioorg. Med. Chem. 16(19) , 8932-9, (2008) Normal and modified urinary nucleosides represent potential biomarkers for cancer diagnosis. To selectively extract modified nucleosides, we developed a molecularly imprinted polymer (MIP) of 5-methyluridine as selective material for molecularly imprinted sol... |