5'-DEOXYTHYMIDINE
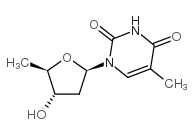
5'-DEOXYTHYMIDINE structure
|
Common Name | 5'-DEOXYTHYMIDINE | ||
|---|---|---|---|---|
| CAS Number | 3458-14-8 | Molecular Weight | 352.13 | |
| Density | 1.344g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C10H13IN2O4 | Melting Point | 192-193ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
|
Thymidine inhibits the growth-arrest-specific degradation of thymidine kinase protein in transfected L fibroblasts.
J. Mol. Biol. 265(2) , 153-60, (1997) The expression of murine thymidine kinase (TK) is strictly dependent on the growth state of the cell. Expressing epitope-tagged TK in LTK cells, we have previously shown that low TK enzyme levels in G0 cells are in part due to a dramatic decrease in TK protei... |
|
|
Membrane permeation characteristics of 5'-modified thymidine analogs.
Mol. Pharmacol. 41(5) , 950-6, (1992) The membrane permeation characteristics of 5'-deoxythymidine (5'-ddThd) and 5'-azido-5'-deoxythymidine (5'-N3-5'-ddThd) were investigated in human erythrocytes, with an inhibitor-stop assay, at 20 degrees. Uptake of both nucleoside analogs occurred without me... |
|
|
Synthesis and HIV-1 reverse transcriptase inhibitor activity of some 2,5,6-substituted benzoxazole, benzimidazole, benzothiazole and oxazolo(4,5-b)pyridine derivatives.
Arzneimittelforschung 53(4) , 266-71, (2003) In this study, the synthesis of some benzoxazoles and their analogues were described and their antiviral activities were studied together with the previously synthesized 2,5,6-trisubstituted benzoxazole, benzothiazole, benzimidazole and oxazolo(4,5-b)pyridine... |
|
|
Formation of aminyl radicals on electron attachment to AZT: abstraction from the sugar phosphate backbone versus one-electron oxidation of guanine.
J. Phys. Chem. B 114(28) , 9289-99, (2010) Employing electron spin resonance (ESR) spectroscopy, we have characterized the radicals formed in 3'-azido-3'-deoxythymidine (3'-AZT) and in its 5'-analog 5'-azido-5'-deoxythymidine (5'-AZT) after electron attachment in gamma-irradiated aqueous (H(2)O or D(2... |
|
|
Study on reactivity and protection of the alpha-hydroxyphosphonate moiety in 5'-nucleotide analogues: formation of the 3'-O-P-C(OH)-C4' internucleotide linkage.
Nucleosides Nucleotides Nucleic Acids 22(3) , 329-47, (2003) The recently described epimeric nucleosidyl-5'-C-phosphonates (alpha-hydroxyphosphonates) represent novel nucleotide analogues that can be incorporated into chimeric oligonucleotides by the phosphotriester condensation method. In order to prepare suitable pro... |
|
|
A-hydroxyphosphonate oligonucleotides: a promising DNA type?
Nucleosides Nucleotides Nucleic Acids 22(5-8) , 1061-4, (2003) The synthesis of monomers (S)-1, (R)-1 and 2 derived from (5'S)-, (5'R)-2'-deoxythymidine-5'-C-phosphonic acids and 2',5'-dideoxythymidine-5'-C-phosphonic acids was elaborated. The protection of the 5'-hydroxyl by the methoxycarbonyl group was a key step of t... |