MRS 1191
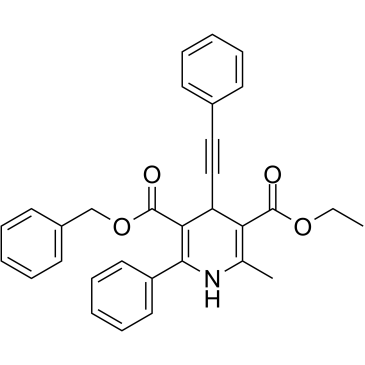
MRS 1191 structure
|
Common Name | MRS 1191 | ||
|---|---|---|---|---|
| CAS Number | 185222-90-6 | Molecular Weight | 477.550 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 643.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C31H27NO4 | Melting Point | 155 - 156 ℃ | |
| MSDS | USA | Flash Point | 342.7±31.5 °C | |
|
ATP releases ATP or other nucleotides from human peripheral blood leukocytes through purinergic P2 receptors.
Life Sci. 145 , 85-92, (2016) Almost every eukaryotic cell releases ATP under certain conditions. The idea that ATP induces the release of ATP has been scantly investigated.We explored this possibility by assessing the rate of exogenous ATP breakdown (measured by phosphates production) by... |
|
|
The infarct-sparing effect of IB-MECA against myocardial ischemia/reperfusion injury in mice is mediated by sequential activation of adenosine A3 and A 2A receptors.
Basic Res. Cardiol. 110(2) , 16, (2015) Conflicting results exist regarding the role of A3 adenosine receptors (A3ARs) in mediating cardioprotection during reperfusion following myocardial infarction. We hypothesized that the effects of the A3AR agonist IB-MECA to produce cardioprotection might inv... |
|
|
Pharmacological characterization of novel A3 adenosine receptor-selective antagonists.
Neuropharmacology 36 , 1157-1165, (1997) The effects of putative A3 adenosine receptor antagonists of three diverse chemical classes (the flavonoid MRS 1067, the 6-phenyl-1,4-dihydropyridines MRS 1097 and MRS 1191, and the triazoloquinazoline MRS 1220) were characterized in receptor binding and func... |
|
|
Activation of hippocampal adenosine A3 receptors produces a desensitization of A1 receptor-mediated responses in rat hippocampus.
J. Neurosci. 17 , 607-614, (1997) The adenosine A3 receptor is expressed in brain, but the consequences of activation of this receptor on electrophysiological activity are unknown. We have characterized the actions of a selective adenosine A3 receptor agonist, 2-chloro-N6-(3-lodobenzyl)-adeno... |
|
|
6-phenyl-1,4-dihydropyridine derivatives as potent and selective A3 adenosine receptor antagonists.
J. Med. Chem. 39 , 4667-4675, (1996) An approach to designing dihydropyridines that bind to adenosine receptors without binding to L-type calcium channels has been described. 1,4-Dihydropyridine derivatives substituted with beta-styryl or phenylethynyl groups at the 4-position and aryl groups at... |