16-hydroxypalmitic acid
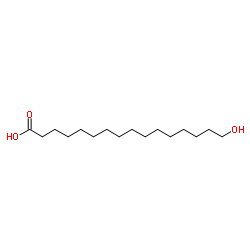
16-hydroxypalmitic acid structure
|
Common Name | 16-hydroxypalmitic acid | ||
|---|---|---|---|---|
| CAS Number | 506-13-8 | Molecular Weight | 272.423 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 414.4±18.0 °C at 760 mmHg | |
| Molecular Formula | C16H32O3 | Melting Point | 94-98 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 218.6±17.7 °C | |
|
Characterization of sophorolipid biosynthetic enzymes from Starmerella bombicola.
FEMS Yeast Res. 15 , (2015) Altering glycolipid structure by genetic engineering of Starmerella bombicola is a recently started research topic and worthy alternative to the unsuccessful selective feeding strategies conventionally applied to reach this goal. One question to be addressed ... |
|
|
CYP77A19 and CYP77A20 characterized from Solanum tuberosum oxidize fatty acids in vitro and partially restore the wild phenotype in an Arabidopsis thaliana cutin mutant.
Plant Cell Environ. , doi:10.1111/pce.12298, (2014) Cutin and suberin represent lipophilic polymers forming plant/environment interfaces in leaves and roots. Despite recent progress in Arabidopsis, there is still a lack on information concerning cutin and suberin synthesis, especially in crops. Based on sequen... |
|
|
Expression of glycine-rich protein genes, AtGRP5 and AtGRP23, induced by the cutin monomer 16-hydroxypalmitic acid in Arabidopsis thaliana.
Plant Physiol. Biochem. 46(11) , 1015-8, (2008) Glycine-rich proteins (GRPs) belong to a large family of heterogenous proteins that are enriched in glycine residues. The expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23, was induced by 16-hydroxypalmitic acid (HPA), a major component ... |
|
|
The omega-hydroxy palmitic acid induced apoptosis in human lung carcinoma cell lines H596 and A549.
J. Biochem. Mol. Biol. Biophys. 6(1) , 37-43, (2002) We have found that omega-hydroxy palmitic acid (16-hydroxy palmitic acid, omega-HPA) has both cell growth inhibiting and cell death inducing actions on human lung adenosquamous carcinoma cell line H596 and adenocarcinoma cell line A549. Further, these effects... |
|
|
Binding of two mono-acylated lipid monomers by the barley lipid transfer protein, LTP1, as viewed by fluorescence, isothermal titration calorimetry and molecular modelling.
Eur. J. Biochem. 268(2) , 384-8, (2001) The binding of two mono-acylated lipid monomers by plant lipid transfer proteins (LTP1s) presents an attractive field of research that could help our understanding of the functional role of this protein family. This task has been investigated in the case of b... |
|
|
An investigation of the likely role of (O-acyl) ω-hydroxy fatty acids in meibomian lipid films using (O-oleyl) ω-hydroxy palmitic acid as a model.
Exp. Eye Res. 115 , 57-64, (2013) (O-acyl) ω-hydroxy fatty acids (OAHFAs) are a recently found group of polar lipids in meibum. Since these lipids can potentially serve as a surfactant in the tear film lipid layer, the surface properties of a molecule of this lipid class was investigated and ... |
|
|
Macrocyclic lactone synthesis by lipases in water-in-oil microemulsions.
Biochim. Biophys. Acta 1257(3) , 239-48, (1995) Five microbial lipases from Chromobacterium viscosum, Candida cylindracea, Pseudomonas (source Fluka), Pseudomonas (source Genzyme) and lipoprotein lipase ex Microbial (Genzyme) have been screened for lactonisation activity towards 16-hydroxyhexadecanoic acid... |
|
|
Defective in cuticular ridges (DCR) of Arabidopsis thaliana, a gene associated with surface cutin formation, encodes a soluble diacylglycerol acyltransferase.
J. Biol. Chem. 285(49) , 38337-47, (2010) A key step in the triacylglycerol (TAG) biosynthetic pathway is the final acylation of diacylglycerol (DAG) by DAG acyltransferase. In silico analysis has revealed that the DCR (defective in cuticular ridges) (At5g23940) gene has a typical HX(4)D acyltransfer... |
|
|
Formation of 20-oxoleukotriene B4 by an alcohol dehydrogenase isolated from human neutrophils.
Biochim. Biophys. Acta 1043(1) , 52-6, (1990) When 20-hydroxyleukotriene B4 (20-OH-LTB4) is incubated at pH 10.5 in the presence of NAD+ with an alcohol dehydrogenase isolated from human neutrophils, a polar product is formed as detected on reverse-phase high-performance liquid chromatography (RP-HPLC). ... |
|
|
Cutin and suberin monomers are membrane perturbants.
J. Colloid. Interface Sci. 271(2) , 507-10, (2004) The interaction between cutin and suberin monomers, i.e., omega -hydroxylpalmitic acid, alpha, omega -hexadecanedioic acid, alpha, omega --hexadecanediol, 12-hydroxylstearic acid, and phospholipid vesicles biomimicking the lipid structure of plant cell membra... |