1-(5-Methyl-2-thienyl)ethanone
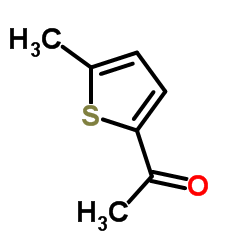
1-(5-Methyl-2-thienyl)ethanone structure
|
Common Name | 1-(5-Methyl-2-thienyl)ethanone | ||
|---|---|---|---|---|
| CAS Number | 13679-74-8 | Molecular Weight | 140.203 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 234.9±20.0 °C at 760 mmHg | |
| Molecular Formula | C7H8OS | Melting Point | 24-28 °C(lit.) | |
| MSDS | USA | Flash Point | 95.8±21.8 °C | |
|
Acylation studies in the thiophene and furan series. IV. Strong inorganic oxyacids as catalysts. Hartough HD and Kosak AI.
J. Am. Chem. Soc. 69(12) , 3093-3096, (1947)
|
|
|
Palladium-catalysed direct 3-or 4-arylation of thiophene derivatives using aryl bromides. Dong JJ, et al.
Tetrahedron 50(23) , 2778-2781, (2009)
|
|
|
Efficient guaiazulene and chamazulene syntheses involving [6+4] cycloadditions. Mukherjee D, et al.
J. Am. Chem. Soc. 1010(1) , 251-252, (1979)
|
|
|
Zinc azaphthalocyanines with thiophen-2-yl, 5-methylthiophen-2-yl and pyridin-3-yl peripheral substituents: Additive substituent contributions to singlet oxygen production. Mørkved EH, et al.
Dyes and Pigments 82(3) , 276-285, (2009)
|
|
|
Synthesis, Configuration, and Antimicrobial Properties of Novel Substituted and Cyclized 2о, 3оо-Thiazachalcones. Ustaa A, et al.
Helv. Chim. Acta 90 , 1482, (2007)
|
|
|
Straightforward access to diketopyrrolopyrrole (DPP) dimers. Stas S, et al.
Dyes and Pigments 97(1) , 198-208, (2013)
|
|
|
Volatile Products Formed from L-Cysteine and Dihydroxyacetone Thermally Treated in Different Solvents. Okumura J, et al.
Agric. Biol. Chem. 54(7) , 1631-1638, (1990)
|
|
|
Novel Dithienylethenes with Extended π‐Systems: Synthesis by Aldol Condensation and Photochromic Properties. Altenhöner K, et al.
European J. Org. Chem. 2010(31) , 6033-6037, (2010)
|
|
|
Experimental thermochemical study of the three methyl substituted 2-acetylthiophene isomers. da Silva MAVR and Santos AFLOM.
J. Chem. Thermodyn. 40(8) , 1309-1313, (2008)
|