1-ethyl-3-methylimidazol-3-ium,acetate
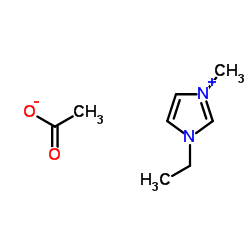
1-ethyl-3-methylimidazol-3-ium,acetate structure
|
Common Name | 1-ethyl-3-methylimidazol-3-ium,acetate | ||
|---|---|---|---|---|
| CAS Number | 143314-17-4 | Molecular Weight | 170.209 | |
| Density | 1.027 g/cm 3 at 25 °C | Boiling Point | N/A | |
| Molecular Formula | C8H14N2O2 | Melting Point | -20℃ | |
| MSDS | Chinese USA | Flash Point | 164ºC | |
|
Determination of solubility parameters of ionic liquids and ionic liquid/solvent mixtures from intrinsic viscosity.
ChemPhysChem 15(16) , 3580-91, (2014) The total and partial solubility parameters (dispersion, polar and hydrogen-bonding solubility parameters) of ten ionic liquids were determined. Intrinsic viscosity approaches were used that encompassed a one-dimensional method (1D-Method), and two different ... |
|
|
Single-step preparation of two-dimensionally organized gold particles via ionic liquid/metal sputter deposition.
Phys. Chem. Chem. Phys. 17 , 13150-9, (2015) Sputtering of noble metals, such as Au, Ag, Pd, and Pt, onto room-temperature ionic liquids (RTILs) enabled the formation of monoparticle films composed of spherical noble metal nanoparticles on the liquid surface only when the RTILs used contained hydroxyl-f... |
|
|
Preparation of corn starch-g-polystyrene copolymer in ionic liquid: 1-ethyl-3-methylimidazolium acetate.
Carbohydr. Polym. 121 , 348-54, (2015) The copolymer of starch grafted with polystyrene (starch-g-PS) was synthesized with high grafting percentage by utilizing the ionic liquid 1-ethyl-3-methylimidazolium acetate ([EMIM]Ac) as solvent and potassium persulfate as initiator. The effect of various p... |
|
|
Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid.
Carbohydr. Polym. 121 , 71-8, (2015) With the serious "white pollution" resulted from the non-biodegradable plastic films, considerable attention has been directed toward the development of renewable and biodegradable cellulose-based film materials as substitutes of petroleum-derived materials. ... |
|
|
Monitoring of cellulose depolymerization in 1-ethyl-3-methylimidazolium acetate by shear and elongational rheology.
Carbohydr. Polym. 117 , 355-63, (2015) The thermal stability of cellulose in the ionic liquid (IL) 1-ethyl-3-methylimidazolium acetate, [emim]OAc was investigated. For this purpose, Eucalyptus urugrandis prehydrolysis kraft pulp was first dissolved in [emim]OAc by means of a vertical kneader and t... |
|
|
Billion-fold enhancement in sensitivity of nuclear magnetic resonance spectroscopy for magnesium ions in solution.
ChemPhysChem 15(18) , 3929-32, (2014) β-nuclear magnetic resonance (NMR) spectroscopy is highly sensitive compared to conventional NMR spectroscopy, and may be applied for several elements across the periodic table. β-NMR has previously been successfully applied in the fields of nuclear and solid... |
|
|
Restricting lignin and enhancing sugar deposition in secondary cell walls enhances monomeric sugar release after low temperature ionic liquid pretreatment.
Biotechnol. Biofuels 8 , 95, (2015) Lignocellulosic biomass has the potential to be a major source of renewable sugar for biofuel production. Before enzymatic hydrolysis, biomass must first undergo a pretreatment step in order to be more susceptible to saccharification and generate high yields ... |
|
|
Interactions of Ionic Liquids with Polysaccharides 1. Unexpected Acetylation of Cellulose with 1-Ethyl-3-methylimidazolium Acetate. Köhler S, et al.
Macromol. Rapid Commun. 28(24) , 2311-2317, (2007)
|
|
|
Hormetic effect of ionic liquid 1-ethyl-3-methylimidazolium acetate on bacteria. Nancharaiah YV and Francis AJ.
Chemosphere 128 , 178-183, (2015)
|
|
|
Bionanocomposite fibers based on cellulose and montmorillonite using ionic liquid 1-ethyl-3-methylimidazolium acetate. Mahmoudian S, et al.
J. Mater. Sci. 50(3) , 1228-1236, (2015)
|