nips hcl
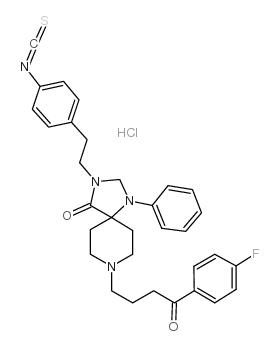
nips hcl structure
|
Common Name | nips hcl | ||
|---|---|---|---|---|
| CAS Number | 135261-88-0 | Molecular Weight | 593.15400 | |
| Density | 1.22g/cm3 | Boiling Point | 754.7ºC at 760mmHg | |
| Molecular Formula | C32H34ClFN4O2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 410.2ºC | |
|
Prolonged D2 antidopaminergic activity of alkylating and nonalkylating derivatives of spiperone in rat brain.
Mol. Pharmacol. 42 , 856-863, (1992) Alkyl and arylalkyl derivatives of the dopamine (DA) D2 antagonist spiperone were prepared and characterized chemically and pharmacologically. They included the N-methyl, N-phenethyl (NPS), and N-p-aminophenethyl (NAPS) derivatives, as well as the alkylating ... |
|
|
N-(p-isothiocyanatophenethyl)spiperone, a selective and irreversible antagonist of D2 dopamine receptors in brain.
J. Pharmacol. Exp. Ther. 257 , 608-615, (1991) N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ), an irreversible and nonselective protein-modifying reagent, has been used extensively in studies involving inactivation of receptors. Here, we present N-(p-isothiocyanatophenethyl)spiperone (NIPS), a nove... |
|
|
Modulation of [(35)S]GTPgammaS binding to chinese hamster ovary cell membranes by D(2(short)) dopamine receptors.
Neurosci. Lett. 280(2) , 135-8, (2000) Rat dopamine D(2short) expressed in Chinese hamster ovary (CHO) cells were characterized by means of activation of [(35)S]-guanosine 5'-O-(gamma-thiotriphosphate) ([(35)S]GTPgammaS) binding and inhibition of [(3)H]raclopride binding. Among 18 dopaminergic lig... |
|
|
Estrogen mediated inhibition of dopamine transport in the striatum: regulation by G alpha i/o.
Eur. J. Pharmacol. 511(2-3) , 121-6, (2005) In the current study, the interaction between estrogen priming and dopamine D2 receptor activation on dopamine uptake in the striatum of ovariectomized female rats was investigated. Basal ADP-[(32)P(i)]ribosylation of G(i/o) was examined in synaptosomal membr... |
|
|
Alteration of dopamine transport in the striatum and nucleus accumbens of ovariectomized and estrogen-primed rats following N-(p-isothiocyanatophenethyl) spiperone (NIPS) treatment.
Brain Res. Bull. 54(6) , 631-8, (2001) The ability of N-(p-isothiocyanatophenethyl) spiperone (NIPS, 10 mg/kg, 24 h), a selective, irreversible alkylating agent of the dopamine D(2) receptor, to alter properties of dopamine uptake and clearance in the striatum and nucleus accumbens of ovariectomiz... |
|
|
Effects of alkylating agents on dopamine D(3) receptors in rat brain: selective protection by dopamine.
Brain Res. 847(1) , 32-7, (1999) Dopamine D(3) receptors are structurally highly homologous to other D(2)-like dopamine receptors, but differ from them pharmacologically. D(3) receptors are notably resistant to alkylation by 1-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ), which readil... |
|
|
Selective alkylatation of dopamine D2 and D4 receptors in rat brain by N-(p-isothiocyanatophenethyl)spiperone.
Neurosci. Lett. 274(3) , 155-8, (1999) Effects of the D2-like receptor alkylating agent NIPS (N-[p-isothiocyanatophenethyl]spiperone) on dopamine receptors in rat brain were characterized by radioreceptor assays and quantitative autoradiography. NIPS alkylated D2 and D4 receptors concentration-dep... |