4,4'-Sulfonyldiphenol
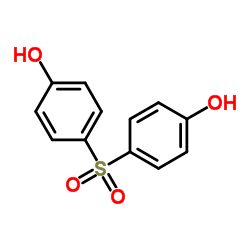
4,4'-Sulfonyldiphenol structure
|
Common Name | 4,4'-Sulfonyldiphenol | ||
|---|---|---|---|---|
| CAS Number | 80-09-1 | Molecular Weight | 250.270 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 505.3±35.0 °C at 760 mmHg | |
| Molecular Formula | C12H10O4S | Melting Point | 245-250 °C(lit.) | |
| MSDS | USA | Flash Point | 259.4±25.9 °C | |
|
Interferences in the direct quantification of bisphenol S in paper by means of thermochemolysis.
J. Chromatogr. A. 1275 , 70-7, (2013) This article analyses the interferences in the quantification of traces of bisphenol S in paper by applying the direct analytical method "analytical pyrolysis gas chromatography mass spectrometry" (Py-GC/MS) in conjunction with on-line derivatisation with tet... |
|
|
Determination of bisphenols in beverages by mixed-mode solid-phase extraction and liquid chromatography coupled to tandem mass spectrometry.
J. Chromatogr. A. 1422 , 230-8, (2015) Facing growing restrictions on the use of bisphenol A in food contact materials, several bisphenol analogs are arising as major alternatives to replace this chemical in most of its applications. This work reports a simple and robust method based on mixed-mode... |
|
|
Derivatization of bisphenol A and its analogues with pyridine-3-sulfonyl chloride: multivariate optimization and fragmentation patterns by liquid chromatography/Orbitrap mass spectrometry.
Rapid Commun. Mass Spectrom. 29 , 1473-84, (2015) Due to the growing restrictions on the use of bisphenol A (BPA), several other bisphenols are gaining importance as substitutes for BPA in a variety of applications. There is, therefore, a real need for selective and sensitive methods based on mass spectromet... |
|
|
Toxicology: The plastics puzzle.
Nature 508(7496) , 306-8, (2014)
|
|
|
Screening of bisphenol A, triclosan and paraben analogues as modulators of the glucocorticoid and androgen receptor activities.
Toxicol. In Vitro 29(1) , 8-15, (2014) A homeostasis of the glucocorticoid and androgen endocrine system is essential to human health. Their disturbance can lead to various diseases, for example cardiovascular, inflammatory and autoimmune diseases, infertility, cancer. Fifteen widely used industri... |
|
|
Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor.
Toxicology 329 , 10-20, (2015) Although much information on the endocrine activity of bisphenol A (BPA) is available, a proper human hazard assessment of analogues that are believed to have a less harmful toxicity profile is lacking. Here the possible effects of BPA, bisphenol F (BPF), bis... |
|
|
Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA).
PLoS ONE 9(10) , e110509, (2014) Bisphenol A (BPA) is an endocrine disrupting environmental contaminant used in a wide variety of products, and BPA metabolites are found in almost everyone's urine, suggesting widespread exposure from multiple sources. Regulatory agencies estimate that virtua... |
|
|
Studies on the interactions of bisphenols with anionic phospholipids of decomposer membranes in model systems.
Biochim. Biophys. Acta 1858 , 756-66, (2016) Bisphenol A (BPA) and other bisphenols constitute a class of organic pollutants, which because of their estrogenic properties, low dose activity and bioaccumulation pose considerable risk for public health as well as for the environment. Accumulated in the se... |
|
|
Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells.
Chemosphere 147 , 19-Sep, (2016) The use of Bisphenol A (BPA) has been regulated in many countries because of its potential adverse effects on human health. As a result of the restriction, structural anologues such as bisphenol S (BPS) and bisphenol F (BPF) have already been used for industr... |
|
|
Use of simple docking methods to screen a virtual library for heteroactivators of cytochrome P450 2C9.
J. Med. Chem. 50 , 1158-65, (2007) Several laboratories have demonstrated that activation of drug metabolism by P450s may occur via a mechanism that resembles allosterism from an enzyme kinetic standpoint. Because the effector drug binding site may be located in the same P450 binding pocket wh... |