1-cyclohexyluracil
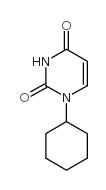
1-cyclohexyluracil structure
|
Common Name | 1-cyclohexyluracil | ||
|---|---|---|---|---|
| CAS Number | 712-43-6 | Molecular Weight | 194.23000 | |
| Density | 1.214g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C10H14N2O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
|
Solvent-dependent photophysics of 1-cyclohexyluracil: ultrafast branching in the initial bright state leads nonradiatively to the electronic ground state and a long-lived 1npi* state.
J. Phys. Chem. B 110(37) , 18641-50, (2006) The modified nucleic acid base, 1-cyclohexyluracil, was studied by femtosecond transient absorption spectroscopy in protic and aprotic solvents of varying polarity. UV excitation at 267 nm populates the lowest-energy bright state, a (1)pipi* state, which has ... |
|
|
Dynamic properties of interaction between nucleic acid bases and models of amino acids.
Biochem. Int. 18(1) , 189-95, (1989) We have discussed the possible hydrogen bonding mode of adenine-uracil base dimers and peptide side chains, together with their characteristic effects on the stability of adenine-uracil base pairing in chloroform solution by using proton nuclear magnetic reso... |
|
|
Fourier transform infrared spectroscopy of 1-cyclohexyluracil aggregates in CDCl(3) solutions.
J. Chem. Phys. 130(12) , 125102, (2009) We reinvestigated the self-aggregation of 1-cyclohexyluracil (1CHU) in CDCl(3) solutions. Wavelength dependent absolute extinction coefficients of the monomer and of dimers are presented on the basis of a simple deconvolution method. Two isomeric dimer struct... |
|
|
Orotic acid decarboxylation in water and nonpolar solvents: a potential role for desolvation in the action of OMP decarboxylase.
Biochemistry 48(36) , 8738-45, (2009) OMP decarboxylase (ODCase) generates a very large rate enhancement without the assistance of metals or other cofactors. The uncatalyzed decarboxylation of 1-methylorotate in water is shown to involve the monoanion, although uncharged 1-methylorotic acid is de... |
|
|
Heteroassociation of O- and N-isopropyl derivatives of barbital and phenobarbital with 9-ethyladenine.
Chem. Biol. Interact. 54(1) , 117-25, (1985) Heteroassociation of O- and N-isopropyl derivatives of barbital and phenobarbital with 9-ethyladenine (9-EA) in CCl4 solutions were studied by infrared spectroscopy. Cyclic heterodimers of high stability (725 less than KH less than 1960 1 X mol-1) compared to... |
|
|
Effect of protein side chain amide group on the hydrogen-bond equilibrium in nucleobases studied by infrared and 13C-NMR spectroscopy.
Biophys. Chem. 34(1) , 1-8, (1989) Infrared spectra of 1:1 hydrogen-bonded complexes formed by derivatives of adenine and model molecules bearing the protein side chain amide group have been measured in chloroform solution. From the temperature dependence of hydrogen-bond formation, thermodyna... |