6-Chloropurineriboside
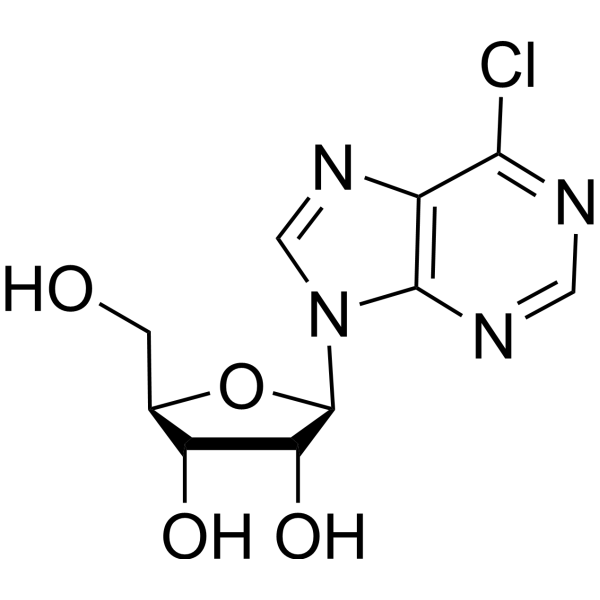
6-Chloropurineriboside structure
|
Common Name | 6-Chloropurineriboside | ||
|---|---|---|---|---|
| CAS Number | 5399-87-1 | Molecular Weight | 286.67 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | 614.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C10H11ClN4O4 | Melting Point | 161-163ºC (dec.) | |
| MSDS | USA | Flash Point | 325.6±34.3 °C | |
|
Evidence on the existence of a purine ligand induced conformational change in the active site of bovine pancreatic ribonuclease A studied by proton nuclear magnetic resonance spectroscopy.
Biochemistry 21(18) , 4290-7, (1982) The titration curves of the C-2 histidine protons of RNase A and of derivative II--a covalent derivative obtained by reaction of the enzyme with the halogenated nucleotide 9-beta-D-ribofuranosyl-6-chloropurine 5'-phosphate--in the presence of a number of puri... |
|
|
Synthesis, biological evaluation and molecular modeling studies of N6-benzyladenosine analogues as potential anti-toxoplasma agents.
Biochem. Pharmacol. 73 , 1558-1572, (2007) Toxoplasma gondii is an opportunistic pathogen responsible for toxoplasmosis. T. gondii is a purine auxotroph incapable of de novo purine biosynthesis and depends on salvage pathways for its purine requirements. Adenosine kinase (EC.2.7.1.20) is the major enz... |
|
|
Synthesis and biological evaluation of nucleoside analogues having 6-chloropurine as anti-SARS-CoV agents.
Bioorg. Med. Chem. Lett. 17 , 2470-3, (2007) Nucleoside analogues that have 6-chloropurine as the nucleobase were synthesized and evaluated for anti-SARS-CoV activity by plaque reduction and yield reduction assays in order to develop novel anti-SARS-CoV agents. Among these analogues, two compounds, name... |
|
|
[Substrate specificity of T4 RNA-ligase: the role of a purine nucleotide base in forming a covalent AMP-RNA-ligase complex].
Biokhimiia 58 , 857-865, (1993) The NTP binding site of bacteriophage T4 RNA-ligase (EC 6.5.1.3) was studied using several ATP analogs modified in the purine moiety of the nucleotide at positions 1, 2, 6 and 8: adenosine-N'-oxide 5'-triphosphate (I), 6-chloropurine riboside 5'-triphosphate ... |
|
|
Introduction of a benzoyl group onto 6-chloropurine riboside in aqueous solution and its application to the synthesis of nucleoside derivatives.
Nucleic Acids Symp. Ser. 44 , 103-104, (2000) A benzoyl group was introduced onto the 3'-hydroxyl group of 6-chloropurine riboside by treatment with benzoic anhydride in the presence of a base in aqueous solution. The product (3b) was converted to 9-(2,3-Di-deoxy-2-fluoro-beta-D-threo-pentofuranosyl)aden... |
|
|
Crystal structure of human type II inosine monophosphate dehydrogenase: implications for ligand binding and drug design.
Proc. Natl. Acad. Sci. U. S. A. 96 , 3531-3536, (1999) Inosine monophosphate dehydrogenase (IMPDH) controls a key metabolic step in the regulation of cell growth and differentiation. This step is the NAD-dependent oxidation of inosine 5' monophosphate (IMP) to xanthosine 5' monophosphate, the rate-limiting step i... |
|
|
Nucleotide binding and affinity labelling support the existence of the phosphate-binding subsite p2 in bovine pancreatic ribonuclease A.
Biochim. Biophys. Acta 953 , 70-78, (1988) When the reaction of bovine pancreatic ribonuclease A with 6-chloropurine riboside 5'-monophosphate was carried out in the presence of several natural mononucleotides, a decrease of 25-75% was found in the amount of the reaction product derivative II (the mai... |
|
|
Purification and characterization of intestinal adenosine deaminase from mice.
Mol. Cell Biochem. 204 , 127-134, (2000) Adenosine deaminase (ADA) was isolated from small intestine of mice and purified to utmost homogeneity. SDS-PAGE of purified ADA gave a molecular weight of 41 kDa. Western blot analyses gave a single reactive band at 41 kDa and the other band was an associate... |
|
|
Lithiation-based silylation and stannylation of 6-chloro-9-(beta-D-ribofuranosyl)purine.
Nucleic Acids Symp. Ser. (34) , 155-6, (1995) Reaction of the lithiated species of 9-(2,3,5-tris-O-tert-butyldimethylsilyl-beta-D-ribofuranosyl)-6-chloropu rine with Me3SiCl or Bu3SnCl was found to furnish the corresponding C-2 substituted product, as a result of silyl or stannyl migration from C-8 to C-... |
|
|
5'-O-masked 2'-deoxyadenosine analogues as lead compounds for hepatitis C virus (HCV) therapeutic agents.
Bioorg. Med. Chem. 15 , 6882-92, (2007) On the basis of our previous study on antiviral agents against the severe acute respiratory syndrome (SARS) coronavirus, a series of nucleoside analogues whose 5'-hydroxyl groups are masked by various protective groups such as carboxylate, sulfonate, and ethe... |