O-SUCCINYL-L-HOMOSERINE
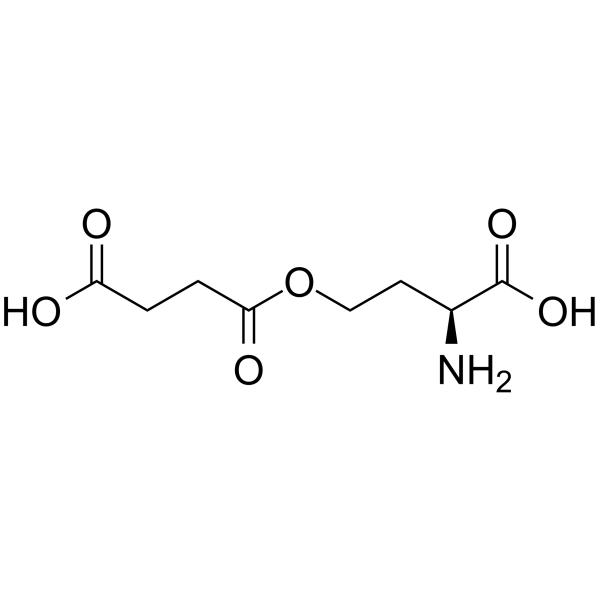
O-SUCCINYL-L-HOMOSERINE structure
|
Common Name | O-SUCCINYL-L-HOMOSERINE | ||
|---|---|---|---|---|
| CAS Number | 1492-23-5 | Molecular Weight | 219.19200 | |
| Density | 1.392g/cm3 | Boiling Point | 492.8ºC at 760mmHg | |
| Molecular Formula | C8H13NO6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 251.8ºC | |
|
The enzymology of cystathionine biosynthesis: strategies for the control of substrate and reaction specificity.
Arch. Biochem. Biophys. 433 , 166-175, (2005) The ability of enzymes to catalyze specific reactions, while excluding others, is central to cellular metabolism. Control of reaction specificity is of particular importance for enzymes that employ catalytically versatile cofactors, of which pyridoxal 5'-phos... |
|
|
Cloning and characterization of two Lactobacillus casei genes encoding a cystathionine lyase.
Appl. Environ. Microbiol. 74 , 99-106, (2008) Volatile sulfur compounds are key flavor compounds in several cheese types. To better understand the metabolism of sulfur-containing amino acids, which certainly plays a key role in the release of volatile sulfur compounds, we searched the genome database of ... |
|
|
Enzymatic characterization and inhibitor discovery of a new cystathionine {gamma}-synthase from Helicobacter pylori.
J. Biochem. 143 , 59-68, (2008) Cystathionine gamma-synthase (CGS) catalyses the first step of the transsulfuration pathway that converts l-cysteine to l-homocysteine in bacteria, whereas this pathway is absent in human. In this report, we identified a new metB gene from Helicobacter pylori... |
|
|
Pathways of assimilative sulfur metabolism in Pseudomonas putida.
J. Bacteriol. 181(18) , 5833-7, (1999) Cysteine and methionine biosynthesis was studied in Pseudomonas putida S-313 and Pseudomonas aeruginosa PAO1. Both these organisms used direct sulfhydrylation of O-succinylhomoserine for the synthesis of methionine but also contained substantial levels of O-a... |
|
|
Cloning and characterization of the CYS3 (CYI1) gene of Saccharomyces cerevisiae.
J. Bacteriol. 174(10) , 3339-47, (1992) A DNA fragment containing the Saccharomyces cerevisiae CYS3 (CYI1) gene was cloned. The clone had a single open reading frame of 1,182 bp (394 amino acid residues). By comparison of the deduced amino acid sequence with the N-terminal amino acid sequence of cy... |
|
|
A direct sulfhydrylation pathway is used for methionine biosynthesis in Pseudomonas aeruginosa.
Microbiology 141 ( Pt 2) , 431-9, (1995) The relationship between genes and enzymes in the methionine biosynthetic pathway has been studied in Pseudomonas aeruginosa. The first step is catalysed by an O-succinylhomoserine synthase, the product of the metA gene mapped at 20 min on the chromosome. The... |
|
|
Crystal structure of Escherichia coli cystathionine gamma-synthase at 1.5 A resolution.
EMBO J. 17(23) , 6827-38, (1998) The transsulfuration enzyme cystathionine gamma-synthase (CGS) catalyses the pyridoxal 5'-phosphate (PLP)-dependent gamma-replacement of O-succinyl-L-homoserine and L-cysteine, yielding L-cystathionine. The crystal structure of the Escherichia coli enzyme has... |
|
|
Reaction mechanism of Escherichia coli cystathionine gamma-synthase: direct evidence for a pyridoxamine derivative of vinylglyoxylate as a key intermediate in pyridoxal phosphate dependent gamma-elimination and gamma-replacement reactions.
Biochemistry 29(2) , 442-51, (1990) Cystathionine gamma-synthase catalyzes a pyridoxal phosphate dependent synthesis of cystathionine from O-succinyl-L-homoserine (OSHS) and L-cysteine via a gamma-replacement reaction. In the absence of L-cysteine, OSHS undergoes an enzyme-catalyzed, gamma-elim... |
|
|
Escherichia coli cystathionine gamma-synthase does not obey ping-pong kinetics. Novel continuous assays for the elimination and substitution reactions.
Biochemistry 42(38) , 11297-306, (2003) Cystathionine gamma-synthase (CGS) is a pyridoxal phosphate-dependent enzyme that catalyzes a gamma-replacement reaction, in which the succinyl group of an O-succinyl-L-homoserine (L-OSHS) is displaced by the thiol of L-cysteine to form L-cystathionine, in th... |
|
|
Direct homocysteine biosynthesis from O-succinylhomoserine in Escherichia coli: an alternate pathway that bypasses cystathionine.
J. Bacteriol. 153(1) , 558-61, (1983) Mutations were found which enable Escherichia coli K-12 to form homocysteine in the absence of cystathionase. The formation of homocysteine in the mutant strains required cystathionine gamma-synthetase, the metB gene product, but bypassed the normal intermedi... |