2-Butenoyl coenzyme A lithium
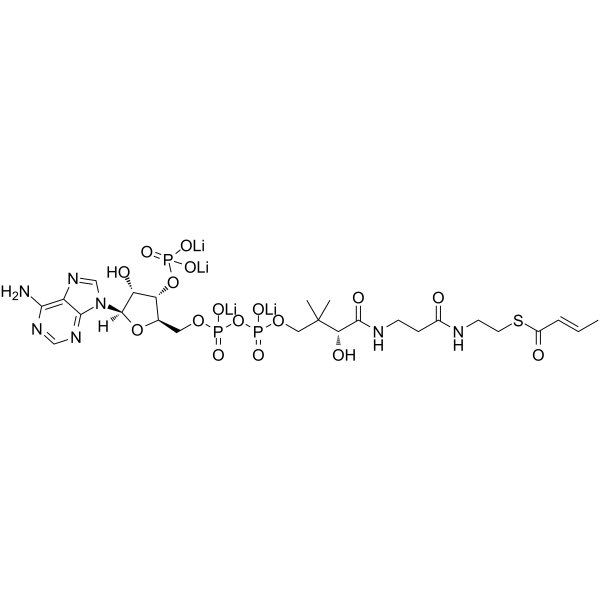
2-Butenoyl coenzyme A lithium structure
|
Common Name | 2-Butenoyl coenzyme A lithium | ||
|---|---|---|---|---|
| CAS Number | 102680-35-3 | Molecular Weight | 835.60800 | |
| Density | 1.82g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C25H40N7O17P3S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
|
Kinetic and structural analysis of the increased affinity of enoyl-ACP (acyl-carrier protein) reductase for triclosan in the presence of NAD+.
Biochem. J. 381 , 725-733, (2004) The binding of enoyl-ACP (acyl-carrier protein) reductase from Plasmodium falciparum (PfENR) with its substrates and inhibitors has been analysed by SPR (surface plasmon resonance). The binding of the substrate analogue crotonoyl-CoA and coenzyme NADH to PfEN... |
|
|
A novel prokaryotic trans-2-enoyl-CoA reductase from the spirochete Treponema denticola.
FEBS Lett. 581 , 1561-1566, (2007) An NADH-dependent trans-2-enoyl-CoA reductase (EC1.1.1.36) from the Gram negative spirochete Treponema denticola was identified, expressed and biochemically characterized. The recombinant protein is a monomeric enzyme with a molecular mass of 44 kDa with a sp... |
|
|
A new beta-hydroxyacyl-acyl carrier protein dehydratase (FabZ) from Helicobacter pylori: Molecular cloning, enzymatic characterization, and structural modeling.
Biochem. Biophys. Res. Commun. 333 , 1078-1086, (2005) Helicobacter pylori is a gram-negative pathogenic bacterium that causes peptic ulcer disease and gastric cancer, and studies of the related potent enzymes associated with this bacterium are urgent for the discovery of novel drug targets. In bacteria, beta-hyd... |
|
|
Design, development, synthesis, and docking analysis of 2'-substituted triclosan analogs as inhibitors for Plasmodium falciparum enoyl-ACP reductase.
IUBMB Life 61 , 1083-1091, (2009) A structure-based approach has been adopted to develop 2'-substituted analogs of triclosan. The Cl at position 2' in ring B of triclosan was chemically substituted with other functional groups like NH(2), NO(2) and their inhibitory potencies against PfENR wer... |