H-Gly-OBZl.HCl
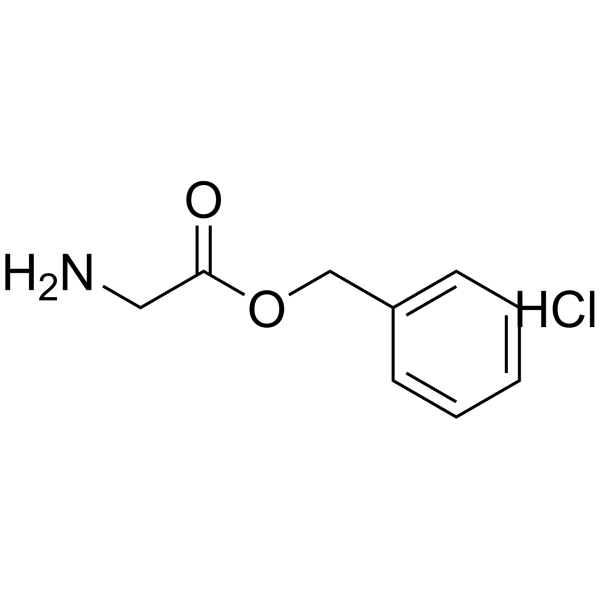
H-Gly-OBZl.HCl structure
|
Common Name | H-Gly-OBZl.HCl | ||
|---|---|---|---|---|
| CAS Number | 2462-31-9 | Molecular Weight | 201.650 | |
| Density | 1.132g/cm3 | Boiling Point | 245.5ºC at 760 mmHg | |
| Molecular Formula | C9H12ClNO2 | Melting Point | 138-140°C | |
| MSDS | USA | Flash Point | 110.3ºC | |
|
The Benzyl Ester Group of Amino Acid Monomers Enhances Substrate Affinity and Broadens the Substrate Specificity of the Enzyme Catalyst in Chemoenzymatic Copolymerization.
Biomacromolecules 17 , 314-23, (2016) The chemoenzymatic polymerization of amino acid monomers by proteases involves a two-step reaction: the formation of a covalent acyl-intermediate complex between the protease and the carboxyl ester group of the monomer and the subsequent deacylation of the co... |
|
|
Synthesis of a metabolite of metoclopramide and its detection in human urine.
Il Farmaco 49 , 805-808, (1994) The synthesis of 2-(4-amino-5-chloro-2-methoxybenzamido)acetic acid 2, a metabolite of metoclopramide 1, has been accomplished through the coupling of 4-amino-5-chloro-2-methoxybenzoic acid 4 with glycine benzyl ester followed by a catalytic hydrogenation. Su... |
|
|
[Lengthening of the peptide chain at the C-terminal end of a glycosylamino acid].
Carbohydr. Res. 50 , 15-22, (1976) An O-glycodipeptide was synthesized by lengthening the peptide chain on the C-terminal side of a glycosylamino acid unit. N-(Benzyloxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)-L-threonine o-nitrophenyl ester and pentachlorophenyl ester were... |