4-Aminophenyl α-D-mannopyranoside
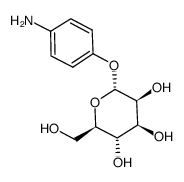
4-Aminophenyl α-D-mannopyranoside structure
|
Common Name | 4-Aminophenyl α-D-mannopyranoside | ||
|---|---|---|---|---|
| CAS Number | 34213-86-0 | Molecular Weight | 271.26600 | |
| Density | 1.517g/cm3 | Boiling Point | 555.9ºC at 760mmHg | |
| Molecular Formula | C12H17NO6 | Melting Point | 165-166ºC | |
| MSDS | USA | Flash Point | 290ºC | |
|
Bovine serum albumin with glycated carboxyl groups shows membrane-perturbing activities.
Arch. Biochem. Biophys. 564 , 43-51, (2014) The aim of the present study aimed to investigate whether glycated bovine serum albumin (BSA) showed novel activities on the lipid-water interface. Mannosylated BSA (Man-BSA) was prepared by modification of the carboxyl groups with p-aminophenyl α-d-mannopyra... |
|
|
Liposomes modified with p-aminophenyl-α-D-mannopyranoside: a promising delivery system in targeting the brain.
Ther. Deliv. 4(12) , 1475-7, (2013)
|
|
|
Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals.
J. Control. Release 141(2) , 183-92, (2010) Chemotherapy for brain glioma has been of limited value due to the inability of transport of drug across the blood-brain barrier (BBB) and poor penetration of drug into the tumor. For overcoming these hurdles, the dual-targeting daunorubicin liposomes were de... |
|
|
Secretory phospholipases A2 induce beta-glucuronidase release and IL-6 production from human lung macrophages.
J. Immunol. 164(9) , 4908-15, (2000) Secretory phospholipases A2 (sPLA2s) are a group of extracellular enzymes that release fatty acids at the sn-2 position of phospholipids. Group IIA sPLA2 has been detected in inflammatory fluids, and its plasma level is increased in inflammatory diseases. To ... |
|
|
Uptake characteristics of liposomes by rat alveolar macrophages: influence of particle size and surface mannose modification.
J. Pharm. Pharmacol. 59(1) , 75-80, (2007) The influence of particle size and surface mannose modification on the uptake of liposomes by alveolar macrophages (AMs) was investigated in-vitro and in-vivo. Non-modified liposomes of five different particle sizes (100, 200, 400, 1000 and 2000 nm) and manno... |
|
|
Affinophoresis of pea lectin and fava bean lectin with an anionic affinophore, bearing rho-aminophenyl-alpha-D-mannoside as an affinity ligand.
J. Chromatogr. A. 400 , 353-9, (1987) Affinophoresis is an electrophoretic separation technique for biological polymers with the aid of an affinophore, which is a macromolecular polyelectrolyte bearing affinity ligands. The affinophore migrates rapidly in an electric field, and consequently the e... |
|
|
Capillary affinophoresis of pea lectin with polyliganded affinophores: a model study of divalent-polyvalent interactions.
Electrophoresis 19(3) , 397-402, (1998) Affinophoresis is a type of affinity electrophoresis using an affinophore, a soluble ionic carrier bearing affinity ligand(s). It was reported previously that an affinophore, prepared by coupling multiple p-aminophenyl alpha-D-mannoside ligands to a part of t... |
|
|
Determination of the affinity constants of pea lectin for neutral sugars by capillary affinophoresis with a monoligand affinophore.
J. Biochem. 120(6) , 1146-52, (1996) Affinophoresis is a type of affinity electrophoresis in which an affinophore, a conjugate of an affinity ligand and a multiply charged soluble matrix, causes a change in migration velocity of proteins which have a specific affinity for the ligand. A monoligan... |
|
|
Liposomes as immunological carriers for the preparation of antimannosyl antibodies.
Experientia 38(5) , 629-30, (1982) Antiserum was raised against an aminophenyl derivative of D-mannose grafted on to a liposomal surface. As characterized by immunodiffusion, quantitative precipitation and hapten inhibition, the antiserum was found to contain mannose specific antibodies in add... |
|
|
Pharmacokinetics and tissue distribution of dual-targeting daunorubicin liposomes in mice.
Pharmacology 87(1-2) , 105-14, (2011) To circumvent the problem of transporting anticancer drugs across the blood-brain barrier (BBB) to target brain tumors, we have previously developed dual-targeting daunorubicin liposomes modified with 4-aminophenyl-α-D-manno-pyranoside and transferrin molecul... |