3-Iodobenzoic acid
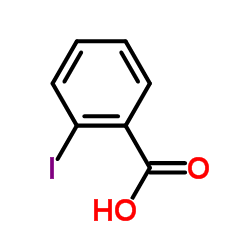
3-Iodobenzoic acid structure
|
Common Name | 3-Iodobenzoic acid | ||
|---|---|---|---|---|
| CAS Number | 618-51-9 | Molecular Weight | 248.018 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | 337.2±25.0 °C at 760 mmHg | |
| Molecular Formula | C7H5IO2 | Melting Point | 185-187 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 157.7±23.2 °C | |
|
Determination of cyclodextrins and their derivatives by capillary electrophoresis with indirect UV and conductivity detection.
Fresenius J. Anal. Chem. 369(7-8) , 666-9, (2001) A fast and simple capillary electrophoretic method suitable for the determination of native alpha-, beta-, gamma-cyclodextrins, their randomly substituted tert-butyl derivatives (average degree of substitution 3.8-4.4), heptakis (2,6-di-O-methyl)- and heptaki... |
|
|
Radioiodination of antibodies via N-succinimidyl 2,4-dimethoxy-3-(trialkylstannyl)benzoates.
Bioconjug. Chem. 1(6) , 387-93, (1990) We have previously shown that use of N-succinimidyl 3-iodobenzoate (SIB) for radioiodination of monoclonal antibodies (MAbs) decreases the loss of radioiodine in vivo compared to MAbs labeled by using conventional methods. Herein, the synthesis of N-succinimi... |
|
|
Negative inotropic effects of Na-salicylate and three congeners on the guinea-pig Langendorff heart.
Arch. Int. Pharmacodyn. Ther. 262(2) , 242-9, (1983) In guinea-pig Langendorff hearts, Na-salicylate (1.9, 3.8 and 7.6 mmol/l) concentration-dependently reduced the contractile force (--9.1, --51.0 and --75.1%, respectively) and the coronary resistance. The influence of the uncoupling agent 2.4-dinitrophenol (0... |
|
|
Photochemical synthesis and properties of 1,6- and 1,8-naphthalenophanes.
Molecules 18(1) , 1314-24, (2013) Various 1,6- and 1,8-naphthalenophanes were synthesized by using the Photo-Dehydro-Diels-Alder (PDDA) reaction of bis-ynones. These compounds are easily accessible from ω-(3-iodophenyl)carboxylic acids in three steps. The obtained naphthalenophanes are axiall... |
|
|
A γ-Turn Mimetic Library: Development and Production. Kocis P, et al.
High-Throughput Synthesis: Principles and Practices New York , (2010), 65
|