Ethyl 3-phenyl-2-propynoate
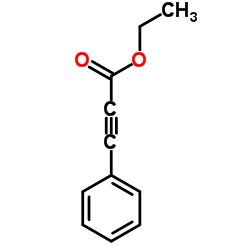
Ethyl 3-phenyl-2-propynoate structure
|
Common Name | Ethyl 3-phenyl-2-propynoate | ||
|---|---|---|---|---|
| CAS Number | 2216-94-6 | Molecular Weight | 174.196 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 265.0±9.0 °C at 760 mmHg | |
| Molecular Formula | C11H10O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 124.9±6.7 °C | |
|
Overexpression of three ubiquitin genes in mouse epidermal tumors is associated with enhanced cellular proliferation and stress.
Cell Growth Differ. 3(5) , 269-78, (1992) A mouse ubiquitin clone that recognizes multiple transcripts overexpressed in murine tumors compared to normal epidermis was isolated by differential screening of complementary DNA libraries from mouse squamous cell carcinomas. Coding region probes detected f... |
|
|
Changes in cytokeratins following treatment of hamster cheek pouch epithelia with hyperplastic or neoplastic agents.
J. Oral. Pathol. Med. 23(4) , 149-55, (1994) The effects of four different hyperplastic agents and of the carcinogen DMBA on cytokeratin expression in hamster cheek pouch epithelia were compared. Reversible hyperplasia was produced by the application of either oil of turpentine, vitamin A or TPA. No hyp... |
|
|
Benzoyl peroxide activation of protein kinase C activity in epidermal cell membranes.
Carcinogenesis 8(12) , 1871-4, (1987) We have investigated the effects of various tumor promoting agents on protein kinase C activity in adult female SENCAR mice. Topical application of benzoyl peroxide increased the calcium-independent activity of protein kinase C in the particulate fraction of ... |
|
|
Induction of ornithine decarboxylase in specific subpopulations of murine epidermal cells following multiple exposures to 12-O-tetradecanoylphorbol-13-acetate, mezerein and ethyl phenylpropriolate.
Carcinogenesis 13(1) , 51-6, (1992) Single applications of 12-O-tetradecanoylphorbol-13-acetate (TPA), mezerein or ethyl phenylpropriolate (EPP) to mouse skin at appropriate doses cause similar degrees of hyperplasia and comparable levels of induction of epidermal ornithine decarboxylase (ODC) ... |
|
|
Non-promoting hyperplasiogenic agents do not mimic the effects of phorbol, 12-myristate, 13-acetate on terminal differentiation of normal and transformed human keratinocytes.
Carcinogenesis 5(5) , 687-90, (1984) We have studied the effects of the potent tumour promoter phorbol, 12-myristate, 13-acetate (PMA) and two non-promoting hyperplasiogenic compounds ethyl phenylpropriolate (EPP) and the divalent cation ionophore A23187 on the terminal differentiation of normal... |
|
|
Comparative histomorphometric changes in SENCAR mouse epidermis in response to multiple treatments with complete and stage-specific tumor promoting agents.
Carcinogenesis 10(10) , 1855-61, (1989) Responses of various cells of the epidermis and dermis to topically applied agents have been implicated in the mechanism of multistage mouse tumorigenesis. These responses have been discussed almost entirely in the context of a single promoter treatment, alth... |
|
|
Tumor-promoting activity of ethyl phenylpropiolate.
Cancer Res. 51(20) , 5642-8, (1991) The ability of the hyperplasiogenic irritant ethyl phenylpropiolate (EPP) to act as a tumor promoter in two-stage carcinogenesis and to stimulate cellular events commonly cited as markers of tumor promoter action was evaluated. Treatment of adult, inbred SENC... |
|
|
Practical and convenient synthesis of coumarins from phenols and propiolic acid esters.
Nat. Protoc. 2(4) , 845-8, (2007) This protocol describes the synthesis of 6,7-methylenedioxy-4-phenylcoumarin from sesamol and ethyl phenylpropiolate using a Pd(OAc)2 catalyst to illustrate coumarin synthesis. This procedure is simple and easy and can be applied to the synthesis of other cou... |
|
|
Rapid promotion and progression of fibrovascular polyps by inflammation and/or hyperplasia in hamster check pouch: implications for carcinogenesis assay.
J. Toxicol. Environ. Health A 11(3) , 467-74, (1983) Tumor initiation by topical application of 7,12-dimethylbenz[a]anthracene (DMBA) in dimethyl sulfoxide (DMSO) followed by topical application of retinyl acetate (RA), ethylphenylpropiolate, or acetic acid in DMSO at inflammatory and hyperplasiogenic dose regi... |
|
|
Local- and systemic-mediated suppression of contact hypersensitivity in mice by several structurally unrelated classes of tumor promoters.
Carcinogenesis 12(10) , 1933-7, (1991) Several structurally unrelated classes of chemicals defined as promoters in the murine skin multistage carcinogenesis protocol were surveyed for their abilities to modify contact hypersensitivity (CHS) responses in SENCAR mice. Sensitization of dorsal skin wi... |