Amprenavir
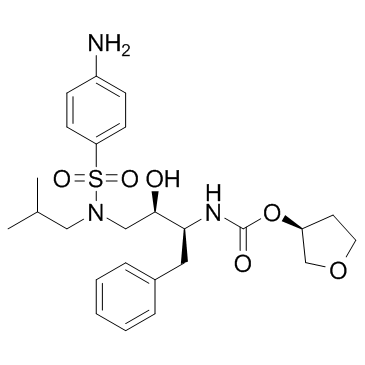
Amprenavir structure
|
Common Name | Amprenavir | ||
|---|---|---|---|---|
| CAS Number | 161814-49-9 | Molecular Weight | 505.627 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 722.5±70.0 °C at 760 mmHg | |
| Molecular Formula | C25H35N3O6S | Melting Point | 72-74ºC | |
| MSDS | Chinese USA | Flash Point | 390.8±35.7 °C | |
|
A comparison of in vitro ADME properties and pharmacokinetics of azithromycin and selected 15-membered ring macrolides in rodents.
Eur. J. Drug Metab. Pharmacokinet. 39(4) , 263-76, (2014) The purpose of this study was to evaluate the impact of structural modifications on the 15-membered macrolactone ring and/or substituents on the in vitro ADME properties and in vivo pharmacokinetic (PK) profile for selected derivatives in rodents in compariso... |
|
|
Parallel ultra high pressure liquid chromatography-mass spectrometry for the quantification of HIV protease inhibitors using dried spot sample collection format.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 965 , 244-53, (2014) An assay was developed and validated for the quantification of eight protease inhibitors (indinavir (IDV), ritonavir (RTV), lopinavir (LPV), saquinavir (SQV), amprenavir (APV), nelfinavir (NFV), atazanavir (AZV) and darunavir (DRV)) in dried plasma spots usin... |
|
|
HZ08 reverse P-glycoprotein mediated multidrug resistance in vitro and in vivo.
PLoS ONE 10(2) , e0116886, (2015) Multidrug efflux transporter P-glycoprotein (P-gp) is highly expressed on membrane of tumor cells and is implicated in resistance to tumor chemotherapy. HZ08 is synthesized and studied in order to find a novel P-gp inhibitor.MDCK-MDR1 monolayer transport, cal... |
|
|
Extreme multidrug resistant HIV-1 protease with 20 mutations is resistant to novel protease inhibitors with P1'-pyrrolidinone or P2-tris-tetrahydrofuran.
J. Med. Chem. 56(10) , 4017-27, (2013) Extreme drug resistant mutant of HIV-1 protease (PR) bearing 20 mutations (PR20) has been studied with the clinical inhibitor amprenavir (1) and two potent antiviral investigational inhibitors GRL-02031 (2) and GRL-0519 (3). Clinical inhibitors are >1000-fold... |
|
|
Suppression of pre adipocyte differentiation and promotion of adipocyte death by anti-HIV drugs.
In Vivo 26(2) , 287-91, (2012) In the present study, we investigated the ability of anti-HIV drugs to interfere with normal cell cycle progression and to induce oxidative stress by perturbing the redox environment. Our results provide evidence that anti-HIV drugs have a differential effect... |
|
|
Role of FAP48 in HIV-associated lipodystrophy.
J. Cell. Biochem. 113(11) , 3446-54, (2012) The highly active antiretroviral therapy (HAART) can cause a metabolic syndrome consisting of lipodystropy/lipoatrophy, dyslipidemia, and type 2 diabetes mellitus with an increased cardiovascular risk. The pathogenetic bases of HAART-associated lipodystrophy ... |
|
|
Nanoparticle-mediated targeted delivery of antiretrovirals to the brain.
Meth. Enzymol. 509 , 41-60, (2012) Nanotechnology offers a new platform for therapeutic delivery of antiretrovirals to the central nervous system (CNS) where human immunodeficiency virus (HIV-1) is sequestered in patients with HIV-1-associated neurological disorders (HAND). HAND is a spectrum ... |
|
|
Pregnane X receptor mediates dyslipidemia induced by the HIV protease inhibitor amprenavir in mice.
Mol. Pharmacol. 83(6) , 1190-9, (2013) Human immunodeficiency virus (HIV) protease inhibitors (PIs) have been used successfully in extending the life span of people infected with HIV. The use of PIs has also been associated with dyslipidemia and an increased risk of cardiovascular disease, but the... |
|
|
Transport inhibition of digoxin using several common P-gp expressing cell lines is not necessarily reporting only on inhibitor binding to P-gp.
PLoS ONE 8(8) , e69394, (2013) We have reported that the P-gp substrate digoxin required basolateral and apical uptake transport in excess of that allowed by digoxin passive permeability (as measured in the presence of GF120918) to achieve the observed efflux kinetics across MDCK-MDR1-NKI ... |
|
|
Differentiation of genotypic resistance profiles for amprenavir and lopinavir, a valuable aid for choice of therapy in protease inhibitor-experienced HIV-1-infected subjects.
J. Antimicrob. Chemother. 52(3) , 319-23, (2003)
|