Triacetin
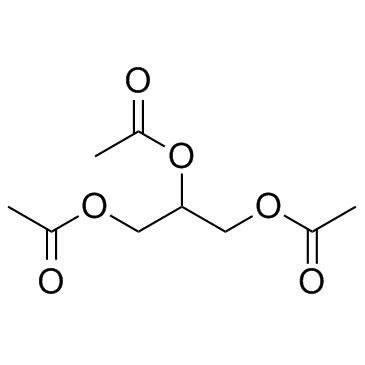
Triacetin structure
|
Common Name | Triacetin | ||
|---|---|---|---|---|
| CAS Number | 102-76-1 | Molecular Weight | 218.204 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 258.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C9H14O6 | Melting Point | 3 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 148.9±0.0 °C | |
|
Biodegradable injectable in situ implants and microparticles for sustained release of montelukast: in vitro release, pharmacokinetics, and stability.
AAPS PharmSciTech 15(3) , 772-80, (2014) The objective of this study was to investigate the sustained release of a hydrophilic drug, montelukast (MK), from two biodegradable polymeric drug delivery systems, in situ implant (ISI) and in situ microparticles (ISM). N-Methyl pyrrolidone (NMP), dimethyl ... |
|
|
Plasticization effect of triacetin on structure and properties of starch ester film.
Carbohydr. Polym. 94(2) , 874-81, (2013) The aim of this work was to evaluate the plasticizing effect of triacetin on the structure and properties of starch ester film and further establish the structure-property relationships. The presence of triacetin resulted in multiple structure changes of the ... |
|
|
Intranasal in situ gel loaded with saquinavir mesylate nanosized microemulsion: preparation, characterization, and in vivo evaluation.
Int. J. Pharm. 475(1-2) , 191-7, (2014) Saquinavir mesylate (SM) is a protease inhibitor with activity against human immunodeficiency virus type 1 (HIV-1) and is available in tablet form, which has three major problems. First, the drug undergoes extensive first pass metabolism. Second, the drug has... |
|
|
Understanding the Mechanism of Enzyme-Induced Formation of Lyotropic Liquid Crystalline Nanoparticles.
Langmuir 31 , 6933-41, (2015) Liquid crystalline nanoparticles have shown great potential for application in fields of drug delivery and agriculture. However, optimized approaches to generating these dispersions have long been sought after. This study focused on understanding the mechanis... |
|
|
Chain length affects pancreatic lipase activity and the extent and pH-time profile of triglyceride lipolysis.
Eur. J. Pharm. Biopharm. 93 , 353-62, (2015) Triglycerides (TG) are one of the most common excipients used in oral lipid-based formulations. The chain length of the TG plays an important role in the oral bioavailability of the co-administered drug. Fatty acid (FA) chain-length specificity of porcine pan... |
|
|
Optimization, ex vivo permeation, and stability study of lipid nanocarrier loaded gelatin capsules for treatment of intermittent claudication.
Int. J. Nanomedicine 10 , 4459-78, (2015) In this study, an optimized nanodispersible oral dosage form (containing a lactate ester) was developed for cilostazol (CZL). CZL is a phosphodiesterase inhibitor used for intermittent claudication. We aimed to improve the dissolution rate and absorption of C... |
|
|
Development and evaluation of new microemulsion-based hydrogel formulations for topical delivery of fluconazole.
AAPS PharmSciTech 16 , 889-904, (2015) The aim of the present investigation was to develop and evaluate microemulsion-loaded hydrogels (MEHs) for the topical delivery of fluconazole (FZ). The solubility of FZ in oils, surfactants and cosurfactants was evaluated to identify the components of the mi... |
|
|
Characterization of a riboflavin non-aqueous nanosuspension prepared by bead milling for cutaneous application.
Chem. Pharm. Bull. 63(2) , 88-94, (2015) The purpose of this study was to characterize the non-aqueous nanosuspension of a hydrophilic drug prepared by bead milling for cutaneous application. Riboflavin was used as the model hydrophilic drug. The non-aqueous nanosuspensions were prepared by grinding... |
|
|
Development and validation of RP-HPLC method: scope of application in the determination of oil solubility of paclitaxel.
J. Chromatogr. Sci. 52(1) , 68-74, (2013) A simple, reproducible, feasible and innovative reversed-phase high-performance liquid chromatographic method was developed and validated for the quantitative determination of paclitaxel dissolved in various oils. The method was validated after extraction of ... |
|
|
In vitro-in vivo evaluation of lipid based formulations of the CETP inhibitors CP-529,414 (torcetrapib) and CP-532,623.
Eur. J. Pharm. Biopharm. 88(3) , 973-85, (2014) The present study investigated the use of lipid based drug delivery systems to enhance the oral bioavailability of the CETP inhibitors CP-532,623 and torcetrapib. A series of self-emulsifying lipid based drug delivery systems (SEDDS) were assembled and examin... |