2-Thiazolecarboxaldehyde
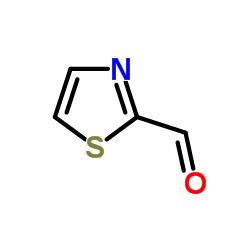
2-Thiazolecarboxaldehyde structure
|
Common Name | 2-Thiazolecarboxaldehyde | ||
|---|---|---|---|---|
| CAS Number | 10200-59-6 | Molecular Weight | 113.138 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 218.3±23.0 °C at 760 mmHg | |
| Molecular Formula | C4H3NOS | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 85.8±22.6 °C | |
|
Design, synthesis, antinociceptive and anti-inflammatory activities of novel piroxicam analogues.
Molecules 17(12) , 14126-45, (2012) In this paper we report the design, synthesis, antinociceptive and anti-inflammatory activities of a series of benzothiazine N-acylhydrazones 14a–h, planned by structural modification of piroxicam (1), a non steroidal anti-inflammatory drug. Among the synthes... |
|
|
Asymmetric synthesis of an N-acylpyrrolidine for inhibition of HCV polymerase.
J. Org. Chem. 73(8) , 3094-102, (2008) A practical asymmetric synthesis of a highly substituted N-acylpyrrolidine on multi-kilogram scale is described. The key step in the construction of the three stereocenters is a [3+2] cycloaddition of methyl acrylate and an imino ester prepared from l-leucine... |
|
|
New highly active taxoids from 9 beta-dihydrobaccatin-9,10-acetals. Part 5.
Bioorg. Med. Chem. Lett. 14(12) , 3209-3215, (2004) To improve the metabolic stability of 3, which exhibited both in vitro antitumor activity and in vivo efficacy by both iv and po administration, we designed and synthesized new taxane analogues. Most of the synthetic compounds maintained excellent antitumor a... |
|
|
Online mechanistic investigations of catalyzed reactions by electrospray ionization mass spectrometry: a tool to intercept transient species in solution. Santos LS.
European J. Org. Chem. 2008(2) , 235-253, (2008)
|
|
|
Synthesis of new thiazole-2,-4, and-5-yl-(amino) methylphosphonates and phosphinates: unprecedented cleavage of thiazole-2 derivatives under acidic conditions. Olszewski TK and Boduszek B.
Tetrahedron 66(45) , 8661-866, (2010)
|
|
|
The Morita-Baylis-Hillman Reaction: Advances and Contributions from Brazilian Chemistry. Santos MS, et al.
Curr. Org. Synth. 12(6) , 830-852, (2015)
|