(r)-(-)-5,5',6,6',7,7',8,8'-octahydro-3,3'-di-t-butyl-1,1'-bi-2-naphthol, dipotassium salt
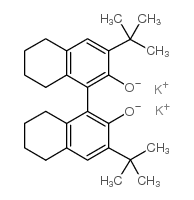
(r)-(-)-5,5',6,6',7,7',8,8'-octahydro-3,3'-di-t-butyl-1,1'-bi-2-naphthol, dipotassium salt structure
|
Common Name | (r)-(-)-5,5',6,6',7,7',8,8'-octahydro-3,3'-di-t-butyl-1,1'-bi-2-naphthol, dipotassium salt | ||
|---|---|---|---|---|
| CAS Number | 350683-75-9 | Molecular Weight | 482.78100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C28H36K2O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
|
A Readily Available and User-Friendly Chiral Catalyst for Efficient Enantioselective Olefin Metathesis This research was supported by the National Institutes of Health (GM-59426) and the National Science Foundation (CHE-9905806 to A. H. H. and CHE-9988766 to R. R. S.).
Angew. Chem. Int. Ed. Engl. 40 , 1452, (2001)
|
|
|
Efficient catalytic enantioselective synthesis of unsaturated amines: preparation of small- and medium-ring cyclic amines through mo-catalyzed asymmetric ring-closing metathesis in the absence of solvent.
J. Am. Chem. Soc. 124 , 6991, (2002) The first catalytic asymmetric ring-closing metathesis method for the synthesis of N-containing heterocycles is reported; this is accomplished through Mo-catalyzed kinetic resolution or desymmetrization of unsaturated amines. Importantly, this catalytic asymm... |
|
|
S.L. Aeilts et al.
Angew. Chem. Int. Ed. Engl. 113 , 1500, (2001)
|
Journals:
More...