a-Homonojirimycin
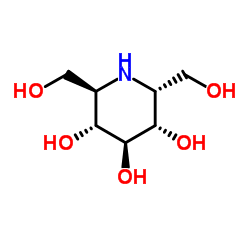
a-Homonojirimycin structure
|
Common Name | a-Homonojirimycin | ||
|---|---|---|---|---|
| CAS Number | 119557-99-2 | Molecular Weight | 193.198 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 432.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C7H15NO5 | Melting Point | 207ºC | |
| MSDS | USA | Flash Point | 224.7±19.3 °C | |
|
In vitro inhibition of glycogen-degrading enzymes and glycosidases by six-membered sugar mimics and their evaluation in cell cultures
Bioorg. Chem. 16 , 7330-7336, (2008) An amyio-1, 6-glucosidase inhibitor enhanced inhibition of hepatic glucose production in combination with glycogen phosphorylase inhibitor. The inhibitory activity of 1-deoxynojirimycin ( 1) toward human maltase was identical to that of voglibose ( 10) of an ... |
|
|
Homonojirimycin analogues and their glucosides from Lobelia sessilifolia and Adenophora spp. (Campanulaceae).
Carbohydr. Res. 323(1-4) , 73-80, (2000) 2,6-Dideoxy-7-O-(beta-D-glucopyranosyl) 2,6-imino-D-glycero-L-gulo- heptitol (7-O-beta-D-glucopyranosyl-alpha-homonojirimycin, 1) was isolated from the 50% methanol extract of the whole plant of Lobelia sessilifolia (Campanulaceae), which was found to potentl... |
|
|
Eight stereoisomers of homonojirimycin from D-mannose.
Org. Lett. 14(8) , 2050-3, (2012) Although there are 32 6-azidoheptitols, there are only 16 homonojirimycin (HNJ) stereoisomers. Two epimeric azidoalditols derived from d-mannose allow the synthesis in water of eight stereoisomers of HNJ.© 2012 American Chemical Society |
|
|
Synthesis and evaluation of glycosidase inhibitory activity of N-butyl 1-deoxy-D-gluco-homonojirimycin and N-butyl 1-deoxy-L-ido-homonojirimycin.
Bioorg. Med. Chem. 14(16) , 5535-9, (2006) Conjugate addition of n-butyl amine to d-glucose derived alpha,beta-unsaturated ester 4 afforded beta-amino esters 5a,b that on reduction of ester group, 1,2-acetonide deprotection, and reductive amination led to the formation of corresponding N-butyl 1-deoxy... |
|
|
Revised structure of a homonojirimycin isomer from Aglaonema treubii: first example of a naturally occurring alpha-homoallonojirimycin.
Bioorg. Med. Chem. Lett. 9(21) , 3171-4, (1999) The structure of a homonojirimycin isomer isolated from Aglaonema treublii and originally proposed as alpha-3,4-di-epi-homonojirimycin was revised to alpha-4-epi-homonojirimycin 3 ("alpha-homoallonojirimycin") on the basis of NMR analysis and synthetic studie... |