heptadecanoic acid tryptamide
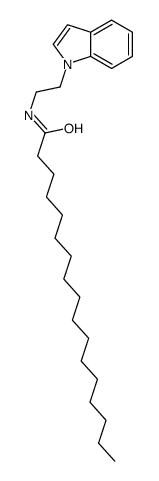
heptadecanoic acid tryptamide structure
|
Common Name | heptadecanoic acid tryptamide | ||
|---|---|---|---|---|
| CAS Number | 232257-97-5 | Molecular Weight | 412.65100 | |
| Density | 0.97g/cm3 | Boiling Point | 585.8ºC at 760 mmHg | |
| Molecular Formula | C27H44N2O | Melting Point | 112-116ºC | |
| MSDS | Chinese USA | Flash Point | 308.1ºC | |
|
Recombinant canine single chain insulin analogues: insulin receptor binding capacity and ability to stimulate glucose uptake.
Vet. J. 202(3) , 436-42, (2014) Virtually all diabetic dogs require exogenous insulin therapy to control their hyperglycaemia. In the UK, the only licensed insulin product currently available is a purified porcine insulin preparation. Recombinant insulin is somewhat problematic in terms of ... |
|
|
Occurrence and spatial distribution of EDCs and related compounds in waters and sediments of Iberian rivers.
Sci. Total Environ. 503-504 , 69-86, (2015) The environmental presence of chemicals capable of affecting the endocrine system has become a matter of scientific and public concern after certain endocrine disruptor compounds (EDCs) have been detected in the aquatic environment. In this work, 31 different... |
|
|
Peptidases compartmentalized to the Ascaris suum intestinal lumen and apical intestinal membrane.
PLoS Negl. Trop. Dis. 9(1) , e3375, (2015) The nematode intestine is a tissue of interest for developing new methods of therapy and control of parasitic nematodes. However, biological details of intestinal cell functions remain obscure, as do the proteins and molecular functions located on the apical ... |
|
|
A transient α-helical molecular recognition element in the disordered N-terminus of the Sgs1 helicase is critical for chromosome stability and binding of Top3/Rmi1.
Nucleic Acids Res. 41(22) , 10215-27, (2013) The RecQ-like DNA helicase family is essential for the maintenance of genome stability in all organisms. Sgs1, a member of this family in Saccharomyces cerevisiae, regulates early and late steps of double-strand break repair by homologous recombination. Using... |
|
|
Low oxygen levels as a trigger for enhancement of respiratory metabolism in Saccharomyces cerevisiae.
BMC Genomics 10 , 461, (2009) The industrially important yeast Saccharomyces cerevisiae is able to grow both in the presence and absence of oxygen. However, the regulation of its metabolism in conditions of intermediate oxygen availability is not well characterised. We assessed the effect... |
|
|
Comparative polygenic analysis of maximal ethanol accumulation capacity and tolerance to high ethanol levels of cell proliferation in yeast.
PLoS Genet. 9(6) , e1003548, (2013) The yeast Saccharomyces cerevisiae is able to accumulate ≥17% ethanol (v/v) by fermentation in the absence of cell proliferation. The genetic basis of this unique capacity is unknown. Up to now, all research has focused on tolerance of yeast cell proliferatio... |
|
|
Regulation of Sphingolipid Biosynthesis by the Morphogenesis Checkpoint Kinase Swe1.
J. Biol. Chem. 291 , 2524-34, (2016) Sphingolipid (SL) biosynthesis is negatively regulated by the highly conserved endoplasmic reticulum-localized Orm family proteins. Defective SL synthesis in Saccharomyces cerevisiae leads to increased phosphorylation and inhibition of Orm proteins by the kin... |
|
|
Genetic variation of Fusarium oxysporum isolates forming fumonisin B(1) and moniliformin.
J. Appl. Genet. 53(2) , 237-47, (2012) Thirty single-spore isolates of a toxigenic fungus, Fusarium oxysporum, were isolated from asparagus spears and identified by species-specific polymerase chain reaction (PCR) and translation elongation factor 1-α (TEF) sequence analysis. In the examined sets ... |
|
|
A novel role of the yeast CaaX protease Ste24 in chitin synthesis.
Mol. Biol. Cell 21(14) , 2425-33, (2010) Ste24 is a membrane-integral CaaX metalloprotease residing in the endoplasmic reticulum (ER). In yeast, the only known substrate of Ste24 is the mating factor a precursor. A global screening for protein-protein interactions indicated that Ste24 interacts with... |
|
|
New CYP17 hydroxylase inhibitors: synthesis, biological evaluation, QSAR, and molecular docking study of new pregnenolone analogs.
Arch. Pharm. (Weinheim) 347(12) , 896-907, (2014) A new series of pregnenonlone analogs were synthesized and evaluated for their inhibitory activity against cytochrome P450 (CYP17 hydroxylase enzyme). In general, the 5-aryl-1,3,4-thiadiazol-2-yl)-imino-pregnenolone derivatives 11-15 were more active than the... |