Dithiobis(2-amino-4-methylpentane)
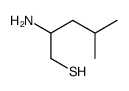
Dithiobis(2-amino-4-methylpentane) structure
|
Common Name | Dithiobis(2-amino-4-methylpentane) | ||
|---|---|---|---|---|
| CAS Number | 88264-65-7 | Molecular Weight | 133.25500 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H15NS | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
|
L-leucinthiol - a potent inhibitor of leucine aminopeptidase.
Biochem. Biophys. Res. Commun. 116 , 297, (1983) L-leucinthiol (2-amino-4-methyl-1-pentanethiol) was designed as an inhibitor of leucine aminopeptidase by analogy with sulfhydryl inhibitors of other zinc-containing peptidases. It was synthesized from L-leucinol and shown to be a potent competitive inhibitor... |
|
|
Synthesis of sulfur-containing analogues of bestatin. Inhibition of aminopeptidases by alpha-thiolbestatin analogues.
J. Med. Chem. 31 , 2193, (1988) Sulfur-containing amino acid and peptide analogues of bestatin [((2S,3R)-3-amino-2-hydroxy-4-phenyl-butanoyl)-L-leucine] (1) have been synthesized and evaluated as inhibitors of aminopeptidase M (AP-M), leucine aminopeptidase (LAP), and aminopeptidase B (AP-B... |
|
|
L-lysinethiol: a subnanomolar inhibitor of aminopeptidase B.
Biochem. Biophys. Res. Commun. 145 , 1038, (1987) L-Lysinethiol was found to be an extremely potent inhibitor of aminopeptidase B (AP-B) with a Ki = 9.1 X 10(-10) M. L-leucinethiol was also a potent inhibitor of AP-B (kj = 1.3 X 10(-7) M), while the D-isomer was much less effective (Ki = 9.8 X 10(-5) M). A t... |
|
|
Structural requirements for specific inhibition of microsomal aminopeptidase by mercaptoamines.
Arch. Biochem. Biophys. 239(2) , 368-74, (1985) L-Leucinthiol, a synthetic derivative of mercaptoethylamine with a hydrophobic side chain, was recently reported to be a potent inhibitor of microsomal aminopeptidase. The structural features necessary for interaction of mercaptoamines with this enzyme have n... |