Isoxicam
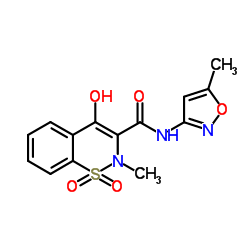
Isoxicam structure
|
Common Name | Isoxicam | ||
|---|---|---|---|---|
| CAS Number | 34552-84-6 | Molecular Weight | 335.335 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 518.8ºC at 760mmHg | |
| Molecular Formula | C14H13N3O5S | Melting Point | 265-271℃ | |
| MSDS | Chinese USA | Flash Point | 267.5ºC | |
|
Effect of some new prostaglandin synthetase inhibitors on the endotoxin induced mortality and biochemical changes in experimental animals.
Res. Commun. Chem. Pathol. Pharmacol. 60(1) , 19-25, (1988) The protective effect of some novel nonsteroidal anti-inflammatory agents has been studied on the endotoxin (lipopolysaccharide B) shock induced mortality in mice and biochemical changes in rats. All the three compounds included in this report, namely isoxica... |
|
|
[The effect of estro-progesterones on the inflammatory episodes of hand arthroses. Apropos of a case].
Rev. Rhum. Mal. Osteoartic. 56(10) , 709-11, (1989)
|
|
|
Controlled-release naproxen compared with isoxicam in patients with osteoarthritis.
Curr. Med. Res. Opin. 11(1) , 28-33, (1988) The therapeutic efficacy and tolerability of a new controlled-release 1000 mg tablet of naproxen (naproxen CR) were compared with 200 mg isoxicam in 100 out-patients with osteoarthritis. Medications were administered once daily for 4 weeks in a controlled, ra... |
|
|
In vivo metabolism of isoxicam in rats, dogs, and monkeys.
Drug Metab. Dispos. 17(6) , 662-8, (1989) Isoxicam is a long half-life nonsteroidal anti-inflammatory agent which undergoes extensive metabolism prior to elimination in animals and man. The major route of isoxicam transformation is hydroxylation of the methylisoxazole functionality to form hydroxymet... |
|
|
Liquid chromatography-tandem mass spectrometry method for the determination of meloxicam and its metabolite 5-carboxymeloxicam in human plasma.
Bioanalysis 1(1) , 63-70, (2009) To develop and validate a rapid, sensitive and selective liquid chromatography-electrospray ionization mass spectrometric method for the determination of meloxicam and its metabolite 5-carboxymeloxicam in human plasma.A liquid extraction method was chosen for... |
|
|
In vitro metabolism of isoxicam by horseradish peroxidase.
Xenobiotica 19(12) , 1369-77, (1989) 1. Disposition studies in vivo in animals and man indicate that hydroxylation of the isoxazole methyl group of isoxicam is the major route of metabolism. 2. Recently, N-methylsaccharin, saccharin, and an open-ring sulphonamide have been identified as addition... |
|
|
Metabolic disposition of the non-steroidal anti-inflammatory agent isoxicam in man.
Eur. J. Drug Metab. Pharmacokinet. 17(1) , 21-7, (1992) The metabolic fate of isoxicam, a long half-life non-steroidal anti-inflammatory agent, in human subjects was investigated using isoxicam labelled with 14C in the N-methyl position. Three healthy male subjects were each administered a single oral 200 mg dose ... |
|
|
Compared effects of isoxicam and indomethacin on the urinary excretion of prostaglandins in degenerative articular diseases.
Prostaglandins Leukot. Essent. Fatty Acids 38(2) , 107-11, (1989) The effects of a 7 day-treatment with isoxicam (200 mg/24 h) on the urinary excretion of prostaglandins (PG) were compared to those of indomethacin (150 mg/24 h) in a double-blind randomized study conducted in 18 patients with degenerative arthritic disease a... |
|
|
[Cytogenetic studies of human lymphocytes under the influence of oxicams].
Z. Rheumatol. 49(2) , 77-81, (1990) The influence of the oxicams, a special group of non-steroidal anti-inflammatory drugs, to the sister chromatid exchange (SCE) was determined on human lymphocytes in vitro and in vivo. The analysis of SCE is a sensitive parameter indicating chromosomal damage... |
|
|
[Severe toxic dermatitis caused by tenoxicam (Tilcotil). 3 cases].
Ann. Dermatol. Venereol. 118(11) , 903-4, (1991)
|