ethyl 3-ethoxy-2-(2,2,2-trifluoroacetyl)acrylate
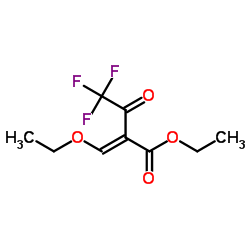
ethyl 3-ethoxy-2-(2,2,2-trifluoroacetyl)acrylate structure
|
Common Name | ethyl 3-ethoxy-2-(2,2,2-trifluoroacetyl)acrylate | ||
|---|---|---|---|---|
| CAS Number | 571-55-1 | Molecular Weight | 240.176 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 211.6±40.0 °C at 760 mmHg | |
| Molecular Formula | C9H11F3O4 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 79.7±22.2 °C | |
|
High-speed microwave-assisted synthesis of the trifluoromethylpyrazol-derived canonical transient receptor potential (TRPC) channel inhibitor Pyr3.
ChemMedChem 4(11) , 1816-8, (2009)
|
|
|
Comparisons of pKa and log P values of some carboxylic and phosphonic acids: synthesis and measurement.
AAPS PharmSci 3(2) , E10, (2001) The changes in the physiochemical properties accompanying the substitution of a phosphonic acid group for a carboxylic acid group on various heterocyclic platforms was determined. A series of low molecular weight heterocyclic carboxylic and phosphonic acids w... |
|
|
Synthesis and cardiotonic activity of 2-substituted 5-cyano-1,6-dihydro-6-oxo-3-pyridinecarboxylic acids and their methyl or ethyl esters.
Il Farmaco 47 , 427, (1992) The synthesis of ethyl or methyl esters of 5-cyano-1,6-dihydro-6-oxo-3- pyridinecarboxylic acids carrying as 2-substituent a polar group such as CO2C2H5, (CH2)2CO2CH3, (CH2)3CO2C2H5, CH2OCH3, or CF3 group is described. Also 2-[5-cyano-1,6-dihydro-2-(1,1-dimet... |
|
|
Novel thiazole-based heterocycles as selective inhibitors of fibrinogen-mediated platelet aggregation.
J. Med. Chem. 38 , 34, (1995) The synthesis and biological activity of novel thiazole-based heterocycles as inhibitors of thrombin-induced human platelet aggregation are described. Further evaluation of selected compounds show they inhibit platelet aggregation as stimulated by a variety o... |
|
|
Synthesis, antiviral (HSV-1) and antimycotic activities of ethyl or methyl 2,4-disubstituted 5-pyrimidinecarboxylates, 2,4-disubstituted 5-pyrimidinecarboxylic acids and 2,4-disubstituted pyrimidines.
Il Farmaco 48 , 335, (1993) The synthesis of ethyl or methyl 4-substituted or unsubstituted 2-methylthio-5-pyrimidinecarboxylates 3 a-i and 8 o mainly by reaction of ethyl or methyl 2-dimethylaminomethylene-3-oxoalkanoates with 2-methylisothiourea is described. Also some ethyl 2-substit... |
|
|
Eur. J. Med. Chem. 28 , 853, (1993)
|