H-Ala-Ala-Ala-OH
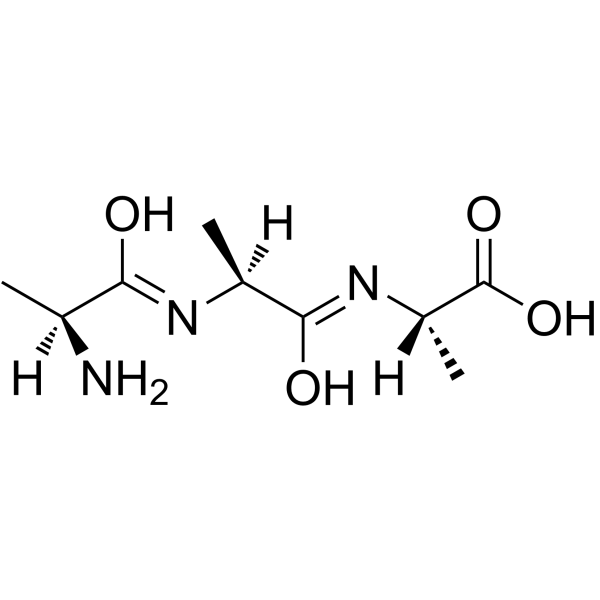
H-Ala-Ala-Ala-OH structure
|
Common Name | H-Ala-Ala-Ala-OH | ||
|---|---|---|---|---|
| CAS Number | 5874-90-8 | Molecular Weight | 231.24900 | |
| Density | 1.227g/cm3 | Boiling Point | 566.5ºC at 760mmHg | |
| Molecular Formula | C9H17N3O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 296.4ºC | |
|
Heating and flooding: a unified approach for rapid generation of free energy surfaces.
J. Chem. Phys. 137(2) , 024102, (2012) We propose a general framework for the efficient sampling of conformational equilibria in complex systems and the generation of associated free energy hypersurfaces in terms of a set of collective variables. The method is a strategic synthesis of the adiabati... |
|
|
Recognition of 2-aminothiazole-4-acetic acid derivatives by the peptide transporters PEPT1 and PEPT2.
Eur. J. Pharm. Sci. 32(1) , 69-76, (2007) The H(+)/peptide cotransporters PEPT1 and PEPT2 have gained considerable interest in pharmaceutical sciences as routes for drug delivery. It is, therefore, of interest to develop uncommon artificial substrates for the two carriers. This study was initiated to... |
|
|
Solvent effects and hydration of a tripeptide in sodium halide aqueous solutions: an in silico study.
Phys. Chem. Chem. Phys. 9(40) , 5423-35, (2007) In this work we are trying to gain insight into the mechanisms of ion-protein interactions in aqueous media at the molecular scale through fully atomistic molecular dynamics simulations. We present a systematic molecular simulation study of interactions of so... |
|
|
Optimization of parameters used in algorithms of ion-mobility calculation for conformational analyses.
J. Phys. Chem. B 114(2) , 1204-12, (2010) Structural information of gaseous ions can be obtained by comparing their collision cross sections as determined by ion-mobility experiments with those by theoretical modeling. Three theoretical models, the projection approximation (PA), the exact hard-sphere... |
|
|
pH-Independence of trialanine and the effects of termini blocking in short peptides: a combined vibrational, NMR, UVCD, and molecular dynamics study.
J. Phys. Chem. B 117(14) , 3689-706, (2013) Several lines of evidence now well establish that unfolded peptides in general, and alanine in specific, have an intrinsic preference for the polyproline II (pPII) conformation. Investigation of local order in the unfolded state is, however, complicated by ex... |
|
|
Quantum-classical description of the amide I vibrational spectrum of trialanine.
J. Chem. Phys. 126(5) , 054509, (2007) A quantum-classical description of the amide I vibrational spectrum of trialanine cation in D2O is given that combines (i) a classical molecular dynamics simulation of the conformational distribution of the system, (ii) comprehensive density functional theory... |
|
|
Molecular mechanics force field-based map for peptide amide-I mode in solution and its application to alanine di- and tripeptides.
Phys. Chem. Chem. Phys. 11(40) , 9149-59, (2009) A molecular mechanics (MM) force field-based empirical electrostatic potential map (MM map) for amide-I vibrations is developed with the aim of seeking a quick and reasonable approach to computing local mode parameters and their distributions in solution phas... |
|
|
Distribution of conformations sampled by the central amino acid residue in tripeptides inferred from amide I band profiles and NMR scalar coupling constants.
J. Phys. Chem. B 113(9) , 2922-32, (2009) The conformational preference of individual amino acid residues in the unfolded state of peptides and proteins is the subject of a continuous debate. Research has mostly been focused on alanine, owing to its abundance in proteins and its relevance for the und... |
|
|
Open-shell pair interaction energy decomposition analysis (PIEDA): formulation and application to the hydrogen abstraction in tripeptides.
J. Chem. Phys. 138(7) , 074111, (2013) An open-shell extension of the pair interaction energy decomposition analysis (PIEDA) within the framework of the fragment molecular orbital (FMO) method is developed. The open-shell PIEDA method allows the analysis of inter- and intramolecular interactions i... |
|
|
Structural analysis of alanine tripeptide with antiparallel and parallel beta-sheet structures in relation to the analysis of mixed beta-sheet structures in Samia cynthia ricini silk protein fiber using solid-state NMR spectroscopy.
J. Am. Chem. Soc. 128(18) , 6231-8, (2006) The structural analysis of natural protein fibers with mixed parallel and antiparallel beta-sheet structures by solid-state NMR is reported. To obtain NMR parameters that can characterize these beta-sheet structures, (13)C solid-state NMR experiments were per... |