D-Cyclohexylalanine
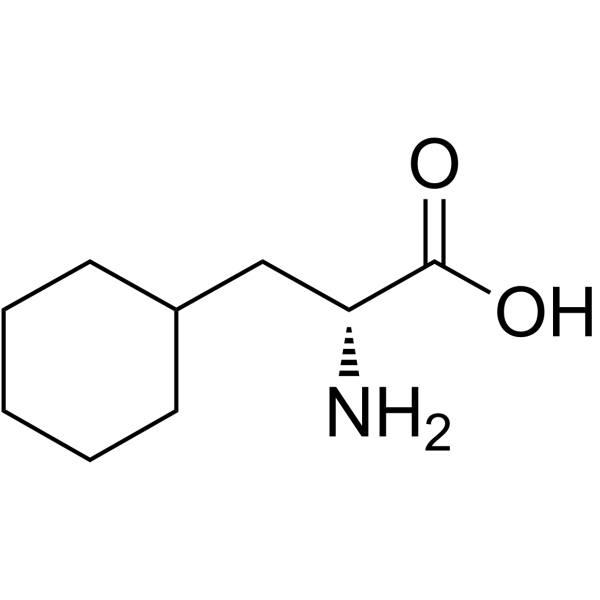
D-Cyclohexylalanine structure
|
Common Name | D-Cyclohexylalanine | ||
|---|---|---|---|---|
| CAS Number | 58717-02-5 | Molecular Weight | 171.237 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 307.1±25.0 °C at 760 mmHg | |
| Molecular Formula | C9H17NO2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 139.6±23.2 °C | |
|
Experimental evaluation of CH-π interactions in a protein core.
Chemistry 18(19) , 5832-6, (2012) CH-π stacks up! Using the protein α(2) D as a model system, we estimate that a CH-π contact between cyclohexylalanine (Cha) and phenylalanine (F) contributes approximately -0.7 kcal mol(-1) to the protein stability. The stacking F-Cha pairs are sequestered i... |
|
|
Sequential and specific exchange of multiple coiled-coil components.
J. Am. Chem. Soc. 125(43) , 13046-51, (2003) The capacity for sequential and specific exchange of single peptides from coiled-coil heterotrimers is investigated. Dual hydrophobic-hydrophilic interface systems permit iterative cycles of pH-triggered strand exchange that can specifically replace one, two,... |
|
|
Mapping the ligand-binding site on the C5a receptor: arginine74 of C5a contacts aspartate282 of the C5a receptor.
Biochemistry 40(46) , 14047-52, (2001) The interaction between the anaphylatoxin C5a and its receptor involves two distinct sites. One site is formed by acidic residues at the receptor N-terminus and contributes to only ligand binding. The second site, responsible for activation, is less well defi... |
|
|
Synthesis and characterization of more potent analogues of hirudin fragment 1-47 containing non-natural amino acids.
Biochemistry 37(39) , 13507-15, (1998) Hirudin is the most potent and specific inhibitor of thrombin, a key enzyme in the coagulation process existing in equilibrium between its procoagulant (fast) and anticoagulant (slow) form. In a previous study, we described the solid-phase synthesis of a Trp3... |
|
|
Up-regulation of adenosine A1 receptor binding in pentylenetetrazol kindling in mice: effects of angiotensin IV.
Brain Res. 1032(1-2) , 94-103, (2005) The effects of the hexapeptide angiotensin II (3-8) ANG IV, the selective A(1) receptor agonist cyclohexyladenosine (CHA) and the combination of ANG IV + CHA on pentylenetetrazol (PTZ)-generalized seizures; kindling development and maintenance were studied. B... |
|
|
Expression levels of adenosine receptors in hippocampus and frontal cortex in argyrophilic grain disease.
Neurosci. Lett. 423(3) , 194-9, (2007) Expression of adenosine receptors of the A1, A2A and A2B type has been examined in the post-mortem frontal cortex and hippocampus in argyrophilic grain disease (AGD), a tauopathy affecting the hippocampus but usually not the frontal cortex, in an attempt to l... |
|
|
The methyl group of N(alpha)(Me)Arg-containing peptides disturbs the active-site geometry of thrombin, impairing efficient cleavage.
J. Mol. Biol. 316(4) , 869-74, (2002) Bivalent peptidic thrombin inhibitors consisting of an N-terminal d-cyclohexylalanine-Pro-N(alpha)(Me)Arg active-site fragment, a flexible polyglycine linker, and a C-terminal hirugen-like segment directed towards the fibrinogen recognition exosite inhibit th... |
|
|
Probing phenylalanine environments in oligomeric structures with pentafluorophenylalanine and cyclohexylalanine.
Biopolymers 95(6) , 410-9, (2011) Stabilization of protein structures and protein-protein interactions are critical in the engineering of industrially useful enzymes and in the design of pharmaceutically valuable ligands. Hydrophobic interactions involving phenylalanine residues play crucial ... |
|
|
Deletion of Ac-NMePhe(1) from [NMePhe(1) ]arodyn under acidic conditions, part 2: effects of substitutions on pharmacological activity.
Biopolymers 96(1) , 103-10, (2011) Arodyn (Ac[Phe¹,²,³,Arg⁴,D-Ala⁸]Dyn A(1-11)NH₂) is an acetylated dynorphin A (Dyn A) analog that is a potent and selective κ opioid receptor antagonist (Bennett et al., J Med Chem 2002, 45, 5617), and its analog [NMePhe¹]arodyn shows even higher affinity and ... |
|
|
Potent noncovalent thrombin inhibitors that utilize the unique amino acid D-dicyclohexylalanine in the P3 position. Implications on oral bioavailability and antithrombotic efficacy.
J. Med. Chem. 40(11) , 1565-9, (1997) In an effort to prepare orally bioavailable analogs of our previously reported thrombin inhibitor 1, we have synthesized a series of compounds that utilize the unique amino acid D-dicyclohexylalanine as a P3 ligand. The resulting compounds are extremely poten... |