(2-Aminophenyl)methanol
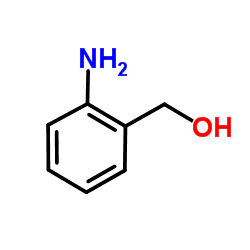
(2-Aminophenyl)methanol structure
|
Common Name | (2-Aminophenyl)methanol | ||
|---|---|---|---|---|
| CAS Number | 5344-90-1 | Molecular Weight | 123.15 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 275.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C7H9NO | Melting Point | 81-83 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 126.3±20.4 °C | |
|
cycloSal-PMEA and cycloAmb-PMEA: potentially new phosphonate prodrugs based on the cycloSal-pronucleotide approach.
J. Med. Chem. 48(25) , 8079-86, (2005) Two new classes of lipophilic prodrugs of the antiviral active phosphonate 9-[2-phosphonomethoxyethyl]adenine (PMEA 1) have been prepared and were studied with regard to their hydrolysis properties and biological activity. A first series of compounds was prep... |
|
|
Heterotrimetallic RuMnMn species on a hydrotalcite surface as highly efficient heterogeneous catalysts for liquid-phase oxidation of alcohols with molecular oxygen.
Angew. Chem. Int. Ed. Engl. 44(22) , 3423-6, (2005)
|
|
|
Facile intramolecular nucleophilic attack by alkoxide ions on ethyl and p-nitrophenyl carbamates.
J. Am. Chem. Soc. 95(11) , 3786-3790, (1973)
|
|
|
In vitro activation of 2-aminobenzyl alcohol and 2-amino-6-nitrobenzyl alcohol, metabolites of 2-nitrotoluene and 2,6-dinitrotoluene.
Chem. Res. Toxicol. 2(3) , 150-6, (1989) Previous results have suggested that key intermediates in the activation of 2-nitrotoluene and 2,6-dinitrotoluene are 2-aminobenzyl alcohol and 2-amino-6-nitrobenzyl alcohol, respectively. In order to determine the metabolic pathway(s) involved in the activat... |
|
|
FT-Raman and FTIR spectra, assignments and ab initio calculations of 2-aminobenzyl alcohol.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 61(3) , 377-85, (2005) The Fourier transform Raman and Fourier transform infrared spectra of 2-aminobenzyl alcohol (2ABA) were recorded in the solid phase. Geometry optimizations were done with out any constraint and harmonic vibrational wave numbers and several thermodynamic param... |
|
|
Ruthenium-catalysed oxidative cyclisation of 2-aminobenzyl alcohol with ketones: modified Friedlaender quinoline synthesis. Cho CS, et al.
Chem. Commun. (Camb.) 24 , 2576-2577, (2001)
|