3-Iodo-L-tyrosine
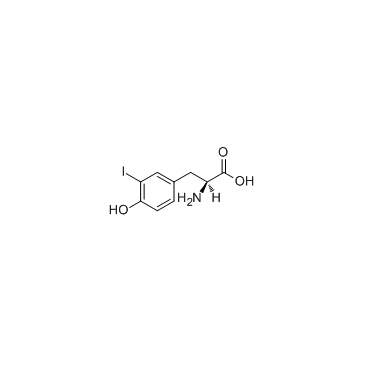
3-Iodo-L-tyrosine structure
|
Common Name | 3-Iodo-L-tyrosine | ||
|---|---|---|---|---|
| CAS Number | 70-78-0 | Molecular Weight | 307.085 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 391.0±42.0 °C at 760 mmHg | |
| Molecular Formula | C9H10INO3 | Melting Point | 210 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 190.3±27.9 °C | |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Impact of hydration state and molecular oxygen on the chemical stability of levothyroxine sodium.
Pharm. Dev. Technol. 20(3) , 314-9, (2015) Levothyroxine sodium is an important medication used primarily for treating patients with hypothyroidism. Levothyroxine sodium tablets have been recalled many times since their 1955 introduction to the US market. These recalls resulted from the failure of lot... |
|
|
Protein stabilization utilizing a redefined codon.
Sci. Rep. 5 , 9762, (2015) Recent advances have fundamentally changed the ways in which synthetic amino acids are incorporated into proteins, enabling their efficient and multiple-site incorporation, in addition to the 20 canonical amino acids. This development provides opportunities f... |
|
|
Brain catecholamine depletion and motor impairment in a Th knock-in mouse with type B tyrosine hydroxylase deficiency.
Brain 138 , 2948-63, (2015) Tyrosine hydroxylase catalyses the hydroxylation of L-tyrosine to l-DOPA, the rate-limiting step in the synthesis of catecholamines. Mutations in the TH gene encoding tyrosine hydroxylase are associated with the autosomal recessive disorder tyrosine hydroxyla... |
|
|
Discovery of {1-[4-(2-{hexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl}-1H-benzimidazol-1-yl)piperidin-1-yl]cyclooctyl}methanol, systemically potent novel non-peptide agonist of nociceptin/orphanin FQ receptor as analgesic for the treatment of neuropathic pain: Design, synthesis, and structure–activity relationships
Bioorg. Med. Chem. 18 , 7675-99, (2010) Neuropathic pain is a serious chronic disorder caused by lesion or dysfunction in the nervous systems. Endogenous nociceptin/orphanin FQ (N/OFQ) peptide and N/OFQ peptide (NOP) receptor [or opioid-receptor-like-1 (ORL1) receptor] are located in the central an... |
|
|
In Vitro, Ex Vivo, and In Vivo Determination of Thyroid Hormone Modulating Activity of Benzothiazoles.
Toxicol. Sci. 146 , 254-64, (2015) As in vitro assays are increasingly used to screen chemicals for their potential to produce endocrine disrupting adverse effects, it is important to understand their predictive capacity. The potential for a set of 6 benzothiazoles to affect endpoints related ... |
|
|
Identification of a potent, selective, and orally active leukotriene a4 hydrolase inhibitor with anti-inflammatory activity.
J. Med. Chem. 51 , 4150-69, (2008) LTA 4H is a ubiquitously distributed 69 kDa zinc-containing cytosolic enzyme with both hydrolase and aminopeptidase activity. As a hydrolase, LTA 4H stereospecifically catalyzes the transformation of the unstable epoxide LTA 4 to the diol LTB 4, a potent chem... |
|
|
The NMDA Receptor Promotes Sleep in the Fruit Fly, Drosophila melanogaster.
PLoS ONE 10 , e0128101, (2015) Considerable evidence indicates that sleep is essential for learning and memory. Drosophila melanogaster has emerged as a novel model for studying sleep. We previously found a short sleeper mutant, fumin (fmn), and identified its mutation in the dopamine tran... |
|
|
Knock-in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current.
J. Neurophysiol. 112(4) , 903-12, (2014) Hundreds of mutations in the SCN1A sodium channel gene confer a wide spectrum of epileptic disorders, requiring efficient model systems to study cellular mechanisms and identify potential therapeutic targets. We recently demonstrated that Drosophila knock-in ... |
|
|
Overexpression of Tyrosine hydroxylase and Dopa decarboxylase associated with pupal melanization in Spodoptera exigua.
Sci. Rep. 5 , 11273, (2015) Melanism has been found in a wide range of species, but the molecular mechanisms involved remain largely elusive. In this study, we studied the molecular mechanisms of the pupal melanism in Spodoptera exigua. The full length cDNA sequences of tyrosine hydroxy... |