cucurbituril
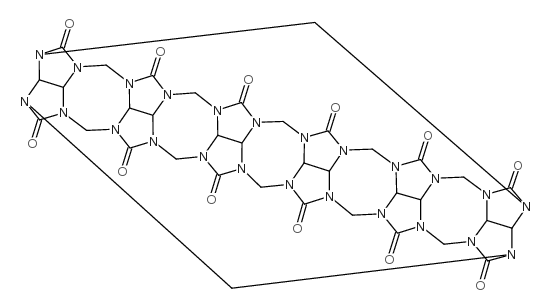
cucurbituril structure
|
Common Name | cucurbituril | ||
|---|---|---|---|---|
| CAS Number | 80262-44-8 | Molecular Weight | 996.82500 | |
| Density | 2.66g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C36H36N24O12 | Melting Point | -470ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
|
Synthesis, processing and solid state excipient interactions of cucurbit[6]uril and its formulation into tablets for oral drug delivery.
Mol. Pharm. 7(6) , 2166-72, (2010) The synthesis, processing, and solid state excipient interactions of cucurbit[6]uril (CB[6]) and its formulation into oral tablets has been examined using a range of physical chemistry techniques. Rapid precipitation from HCl by the addition of water yields m... |
|
|
Unusual complex formation and chemical reaction of haloacetate anion on the exterior surface of cucurbit[6]uril in the gas phase.
J. Am. Soc. Mass Spectrom. 23(10) , 1786-93, (2012) Noncovalent interactions of cucurbit[6]uril (CB[6]) with haloacetate and halide anions are investigated in the gas phase using electrospray ionization ion mobility mass spectrometry. Strong noncovalent interactions of monoiodoacetate, monobromoacetate, monoch... |
|
|
An intelligent anticorrosion coating based on pH-responsive supramolecular nanocontainers.
Nanotechnology 23(50) , 505705, (2012) The hollow mesoporous silica nanoparticles (HMSNs), which have been used as the nanocontainers for the corrosion inhibitor, benzotriazole, were fabricated using the hard-template method. Alkaline-responsive HMSNs based on cucurbit[6]uril (CB[6])/bisammonium s... |
|
|
Study on the inclusion interaction of cucurbit[n]urils with sanguinarine by spectrofluorimetry and its analytical application.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 75(2) , 912-7, (2010) The characteristics of host-guest complexation between cucurbit[n]uril (CB[n], n=5, 6, 7, 8) and sanguinarine (SA) were investigated by spectrofluorimetry. The result showed that CB[n] (n=5, 6, 7, 8) reacted with SA to form an inclusion complex. At the optimu... |
|
|
Glyco-pseudopolyrotaxanes: carbohydrate wheels threaded on a polymer string and their inhibition of bacterial adhesion.
Chemistry 16(40) , 12168-73, (2010) We report glyco-pseudopolyrotaxanes composed of cucurbit[6]uril-based mannose wheels (ManCB[6]) threaded on polyviologen (PV), which not only effectively induce bacterial aggregation, but also exhibit high inhibitory activity against bacterial binding to host... |
|
|
Binding studies on CB[6] with a series of 1-alkyl-3-methylimidazolium ionic liquids in an aqueous system.
Chem. Asian J. 5(3) , 530-7, (2010) The host-guest chemistry between a series of 1-alkyl-3-methyl-imidazolium bromide ([C(n)mim]Br) guests and the macrocyclic host molecule cucurbit[6]uril (CB[6]) in an aqueous system is systematically studied in neutral aqueous media. Both 1D and 2D NMR experi... |
|
|
Supramolecular shuttle based on inclusion complex between cucurbit[6]uril and bispyridinium ethylene.
Org. Lett. 13(23) , 6148-51, (2011) Cucurbit[6]uril (CB6) and bispyridinium ethylene form a stable inclusion complex. A rotaxane derived from this complex was prepared in which a CB6 wheel shuttles along an axle in an NMR time-resolved regime. |
|
|
Complexation of cyclohexanocucurbit[6]uril with cadmium ions: X-ray crystallographic and electrochemical study.
Inorg. Chem. 49(17) , 7638-40, (2010) Complexation of cyclohexanocucurbit[6]uril (Q*[6]), a water-soluble cucurbit[n]uril derivative, with Cd(2+) ions has been studied by means of cyclic voltammetry and differential pulse voltammetry. The electrochemical experimental data prove the formation of a... |
|
|
Controlled catch and release of small molecules with cucurbit[6]uril via a kinetic trap.
Chem. Commun. (Camb.) (22) , 3243-5, (2009) For the first time a lid-free and charge-free inclusion complex with cucurbit[6]uril (CB[6]) has been isolated, the crystal structure is reported and the robust nature of the complex is demonstrated in the solid state while facile controlled release of the gu... |
|
|
End-to-end distance determination in a cucurbit[6]uril-based rotaxane by PELDOR spectroscopy.
ChemPhysChem 13(11) , 2659-61, (2012) Distance determination: The use of pulsed electron-electron double resonance (PELDOR/DEER) spectroscopy to determine the distances among the end units of a paramagnetic cucurbit[6]uril-based rotaxane is demonstrated.Copyright © 2012 WILEY-VCH Verlag GmbH & Co... |