HARMALOL HYDROCHLORIDE DIHYDRATE
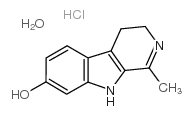
HARMALOL HYDROCHLORIDE DIHYDRATE structure
|
Common Name | HARMALOL HYDROCHLORIDE DIHYDRATE | ||
|---|---|---|---|---|
| CAS Number | 6028-00-8 | Molecular Weight | 254.71300 | |
| Density | N/A | Boiling Point | 591.4ºC at 760 mmHg | |
| Molecular Formula | C12H15ClN2O2 | Melting Point | 265-268ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 311.5ºC | |
|
Fast determination of harmala alkaloids in edible algae by capillary electrophoresis mass spectrometry.
Anal. Bioanal. Chem 407(13) , 3637-45, (2015) The use of algae as a foodstuff is rapidly expanding worldwide from the East Asian countries, where they are also used for medical care. Harmala alkaloids (HAlk) are a family of bioactive compounds found in the extracts of some plants, including wakame (Undar... |
|
|
Interaction of β-carboline alkaloids with RNA.
DNA Cell. Biol. 29 , 753-761, (2010) β-Carboline alkaloids are present in medicinal plants such as Peganum harmala L., which have been used as folk medicine in anticancer therapy. Recently, they have drawn attention because of their antitumor activities. Despite considerable interest and investi... |
|
|
Beta-carboline alkaloids bind DNA.
J. Photochem. Photobiol. B, Biol. 100 , 84-91, (2010) Beta-carboline alkaloids present in Peganum harmala (harmal) have recently drawn attention due to their antitumor activities. The mechanistic studies indicate that beta-carboline derivatives inhibit DNA topoisomerases and interfere with DNA synthesis. They in... |
|
|
Beta-carboline alkaloids harmaline and harmalol induce melanogenesis through p38 mitogen-activated protein kinase in B16F10 mouse melanoma cells.
BMB Rep. 43 , 824-829, (2010) Melanin synthesis is regulated by melanocyte specific enzymes and related transcription factors. _-carboline alkaloids including harmaline and harmalol are widely distributed in the environment including several plant families and alcoholic beverages. Present... |
|
|
Harmine is a potent antimalarial targeting Hsp90 and synergizes with chloroquine and artemisinin.
Antimicrob. Agents Chemother. 56(8) , 4207-13, (2012) Previous studies have shown an antimalarial effect of total alkaloids extracted from leaves of Guiera senegalensis from Mali in West Africa. We independently observed that the beta-carboline alkaloid harmine obtained from a natural product library screen inhi... |
|
|
Harmaline and harmalol inhibit the carcinogen-activating enzyme CYP1A1 via transcriptional and posttranslational mechanisms.
Food Chem. Toxicol. 50(2) , 353-62, (2012) Dioxins are known to cause several human cancers through activation of the aryl hydrocarbon receptor (AhR). Harmaline and harmalol are dihydro-β-carboline compounds present in several medicinal plants such as Peganum harmala. We have previously demonstrated t... |
|
|
Combined fluorescence spectroscopy and molecular modeling studies on the interaction between harmalol and human serum albumin.
J. Pharm. Biomed. Anal. 67-68 , 201-8, (2012) The interaction between harmalol and human serum albumin (HSA) has been studied by fluorescence spectroscopy and molecular modeling methods. The intrinsic fluorescence of HSA was quenched by harmalol, which was rationalized in terms of the static quenching me... |
|
|
Inhibitory effect of hydroxyindoles and their analogues on human melanoma tyrosinase.
Z. Naturforsch., C, J. Biosci. 65 , 49-54, (2010) A recent study showed that N-acylserotonin derivatives have strong inhibitory activity against tyrosinase. To clarify the role of the 5-hydroxy group in the indole ring, 2-, 4-, 5-, 6-, and 7-hydroxyindole and 11 related compounds such as 5-hydroxyindan and 6... |
|
|
HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L.
J. Pharm. Biomed. Anal. 31(2) , 263-9, (2003) A simple and sensitive method for separation and determination of harmol, harmalol, harmine and harmaline has been developed and validated. Harmol, harmalol, harmine and harmaline were separated using a Metasil ODS column by isocratic elution with flow rate 1... |
|
|
Synthesis and characterization of novel biologically active platinum(II) and palladium(II) complexes of some β-carboline alkaloids
J. Inorg. Biochem. 38(1) , 47-56, (1990) The preparation of novel biologically active platinum(II) and palladium(II) complexes of some β-carboline alkaloids (harmaline, harmalol, harmine, and harmane) is described. These complexes, characterized on the basis of their CHN elemental analysis, infrared... |