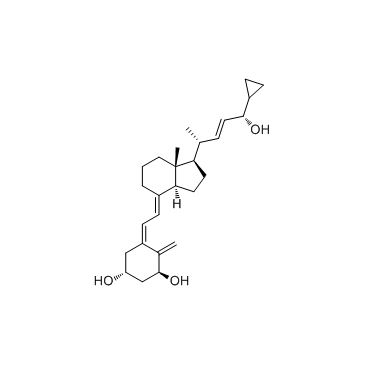
112965-21-6
| 中文名 | 卡泊三醇搽剂 |
|---|---|
| 英文名 | calcipotriol |
| 中文别名 |
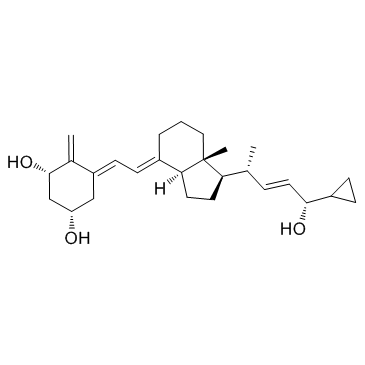
(1a,3b,5z,7e,22e,24s)-24-环丙基-9,10-开环胆甾-5,7,10(19),22-四烯-1,3,24-三醇
钙泊三醇 达力士 |
| 英文别名 |
(1R,3S,5Z)-5-[(2E)-2-{(1R,3aS,7aR)-1-[(1R,2E,4S)-4-cyclopropyl-4-hydroxy-1-méthylbut-2-én-1-yl]-7a-méthyloctahydro-4H-indén-4-ylidène}éthylidène]-4-méthylidènecyclohexane-1,3-diol
(1R,3S,5Z)-5-[(2E)-2-{(1R,3aS,7aR)-1-[(1R,2E,4S)-4-cyclopropyl-4-hydroxy-1-methylbut-2-en-1-yl]-7a-methyloctahydro-4H-inden-4-ylidene}ethylidene]-4-methylidenecyclohexane-1,3-diol Didrogyl Calcipotriol hydrate 25-HYDROXYCHOLECALCIFEROL Calcifediol 25-HYDROXYVITAMIN D3 Calcipotriene CALCIPOTRIENE,EP5.3 VITAMIN D3,25-HYDROXY (1R,3S,5Z)-5-[(2E)-2-{(1R,3aS,7aR)-1-[(1R,2E,4S)-4-Cyclopropyl-4-hydroxy-1-methylbut-2-en-1-yl]-7a-methyloctahydro-4H-inden-4-yliden}ethyliden]-4-methylidencyclohexan-1,3-diol 25-OH vitamin D3 MFCD00866630 (1S,3R,5E,7E,22E,24S)-26,27-Cyclo-9,10-secocholesta-5,7,10,22-tetraene-1,3,24-triol Psorcutan Calcipotriol Hidroferol (1S,3R,5Z,7E,22E,24S)-26,27-Cyclo-9,10-secocholesta-5,7,10,22-tetraene-1,3,24-triol CALCIDIOL (5Z,7E,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol |
| 描述 | Calcipotriol 是一种合成的 VitD3 类似物,对 vitamin D 受体具有高亲和力。 |
|---|---|
| 相关类别 | |
| 靶点 |
Vitamin D receptor[1] |
| 体外研究 | 当NHEK细胞不用IL-17A或IL-22刺激时,卡泊三醇略微增强(0.2nM)IL-8mRNA表达或没有效果(2-20nM)。 IL-17A和IL-22的加入显着增加了IL-8的mRNA表达,证实了我们之前的研究。卡泊三醇在2,20和40 nM时以剂量依赖的方式抑制这种增强的IL-8 mRNA表达[1]。用药物处理天然杀伤(NK)细胞调节它们的NK细胞毒性受体或KIR的表达。用100,10或1ng/mL的1,25(OH)2D3,Calcipotriol或FTY720预处理人NK细胞4小时。所有三种浓度的1,25(OH)2D3,Calcipotriol和FTY720在孵育4小时后显着上调NKp30表面NKp30的表达[2]。 |
| 体内研究 | 除Diclofenac加DFMO加Calcipotriol组外,每组中32只动物中有1只死亡,其中所有动物均存活。生存在各组之间平均分配。与安慰剂(线性回归模型)相比,用双氯芬酸加卡泊三醇(p = 0.018)和双氯芬酸加DFMO加卡泊三醇(p = 0.002)治疗组的体重增加显着较小[3]。 |
| 细胞实验 | 正常人表皮角质形成细胞(NHEK)在无血清角质形成细胞生长培养基Epilife中生长,并在所有实验中在第三代使用。在实验前48小时省略生长补充剂。作为对照,将IL-17A和IL-22加入或不加入细胞中。用IL-17A(200ng / mL)和/或IL-22(200ng / mL)刺激培养的NHEK细胞,然后在0.2-40nM存在或不存在Calcipotriol的情况下共孵育以测试其调节作用。 3天后收获细胞并进行实时定量PCR(qPCR)。还收集培养物上清液并在-80℃冷冻直至用于ELISA [1]。 |
| 动物实验 | 小鼠[3]使用160只雌性SKH-1无毛小鼠(6-7周龄)。在UV处理后,将没有肿瘤的小鼠随机分成五组,四个化学预防组(双氯芬酸加DFMO;双氯芬酸加卡泊三醇; DFMO加骨化三醇;和双氯芬酸加DFMO加卡泊三醇)和一个安慰剂组(皮肤洗剂)。本研究中使用的安慰剂组与早期研究中使用的相同。每天一次,每周五天,用测试混合物处理小鼠,共17周。将测试混合物局部施用于小鼠的背部表面。用移液管施加10微升,然后将混合物摩擦到皮肤上。这相当于治疗中每种活性物质的以下剂量:双氯芬酸100μg/周(未稀释30 mg / g),骨化三醇0.166μg/周(未稀释50μg/ g),二氟甲基鸟氨酸463.3μg/周( DFMO)(139mg / g未稀释)。 |
| 参考文献 |
| 密度 | 1.1±0.1 g/cm3 |
|---|---|
| 沸点 | 582.0±50.0 °C at 760 mmHg |
| 熔点 | 166-168ºC |
| 分子式 | C27H40O3 |
| 分子量 | 412.605 |
| 闪点 | 250.6±24.7 °C |
| 精确质量 | 412.297760 |
| PSA | 60.69000 |
| LogP | 5.43 |
| 外观性状 | 白色结晶固体 |
| 蒸汽压 | 0.0±3.7 mmHg at 25°C |
| 折射率 | 1.580 |
| 储存条件 | Desiccate at -20°C |
| 分子结构 | 1、 摩尔折射率:122.17 2、 摩尔体积(cm3/mol):367.2 3、 等张比容(90.2K):967.5 4、 表面张力(dyne/cm):48.1 5、 极化率(10-24cm3):48.43 |
| 更多 | 1.性状:从甲酸甲酯中结晶。 2.熔点(℃):166~168。 3.UV最大吸收(96%乙醇):264nm(ε17200) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 上游产品 10 | |
|---|---|
| 下游产品 0 | |



![[3S-(1Z,3a,5b)]-[2-[3,5-二[(叔丁基)二甲基硅氧基]-2-亚甲基环己亚基]乙基]二苯基氧化膦结构式](https://image.chemsrc.com/caspic/143/81522-68-1.png)