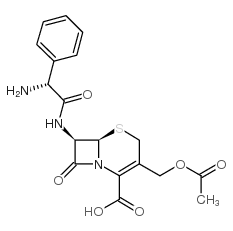
3577-01-3
| 中文名 | 头孢来星 |
|---|---|
| 英文名 | cefaloglycin |
| 英文别名 |
Cefaloglycin
Cephaloglycine (6R)-3-acetoxymethyl-7t-((R)-2-amino-2-phenyl-acetylamino)-8-oxo-(6rH)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid Cephaoglycin acid (6R,7R)-3-(acetyloxymethyl)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Cefaloglycine Cefaloglicina CEPHALOGLYCIN D-Cephaloglycine Kafocin Cefaloglycinum Cephaloglycin anhydrous |
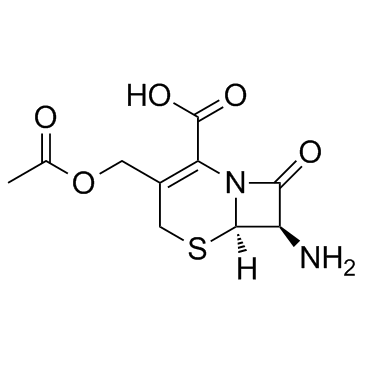
| 描述 | Cefaloglycin (Cephaloglycin) 是一种具有口服活性的肾毒性 β-内酰胺类 (β-lactam) 头孢菌素抗生素,具有抗菌活性。Cefaloglycin 对肠球菌以外的革兰氏阳性球菌 (Gram-Positive cocci) 有活性。Cefaloglycin 对线粒体底物的摄取和呼吸也具有毒性。 |
|---|---|
| 相关类别 | |
| 靶点 |
Gram-Positive cocci[2] |
| 体外研究 | 头孢克洛在体外抗化脓链球菌的活性方面与头孢菌素相当,并略优于头孢氨苄[2]。 |
| 体内研究 | 在每毫升暴露2至6小时至300至3000微克头孢甘氨酸(头孢甘氨酸)的兔肾皮质线粒体中,测量琥珀酸盐和ADP的呼吸和摄取,然后清洗以去除抗生素。头孢菌素(Cefaloglycin)不可逆地降低呼吸和琥珀酸摄取。头孢氨苄对线粒体底物摄取和呼吸的体外损伤模式具有时间依赖性和浓度依赖性[1]。 |
| 参考文献 |
| 密度 | 1.52 g/cm3 |
|---|---|
| 沸点 | 761.8ºC at 760mmHg |
| 熔点 | 236.5°C (rough estimate) |
| 分子式 | C18H19N3O6S |
| 分子量 | 405.42500 |
| 闪点 | 414.5ºC |
| 精确质量 | 405.09900 |
| PSA | 164.33000 |
| LogP | 1.01720 |
| 折射率 | 1.678 |
| 储存条件 | 库房通风低温干燥 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
3577-01-3 |
| 文献:Ogasa; Saito; Hashimoto; Sato; Hirata Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 2 p. 315 - 321 |
|
~% 
3577-01-3 |
| 文献:Spencer; Flynn; Roeske; Siu; Chauvette Journal of medicinal chemistry, 1966 , vol. 9, # 5 p. 746 - 750 |
|
~% 
3577-01-3 |
| 文献:Spencer; Flynn; Roeske; Siu; Chauvette Journal of medicinal chemistry, 1966 , vol. 9, # 5 p. 746 - 750 |
|
~% 
3577-01-3 |
| 文献:Kawamori; Hashimoto; Katsumata; et al. Agricultural and Biological Chemistry, 1983 , vol. 47, # 11 p. 2503 - 2509 |
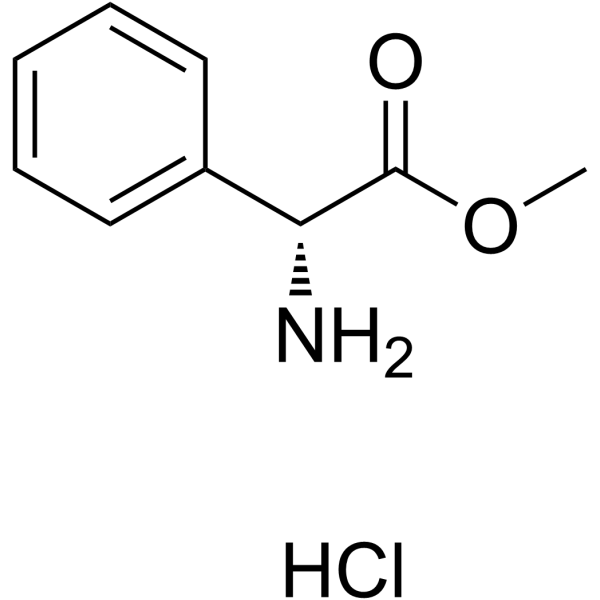
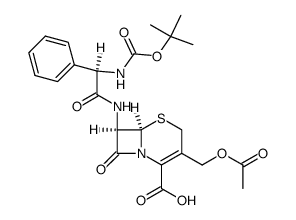
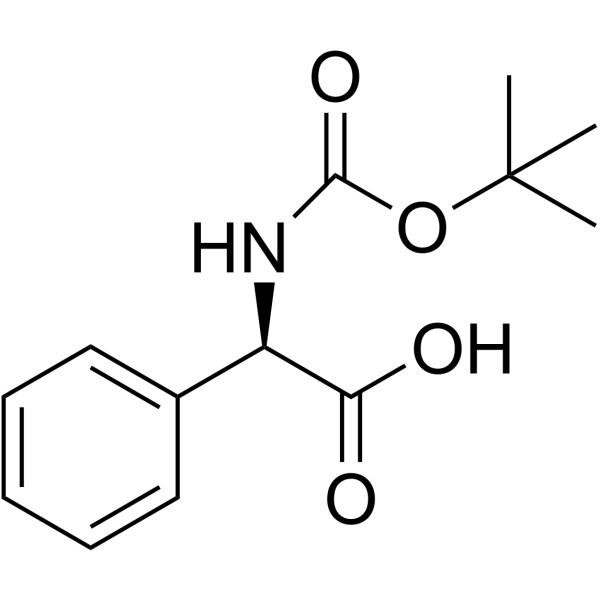
| 上游产品 5 | |
|---|---|
| 下游产品 0 | |