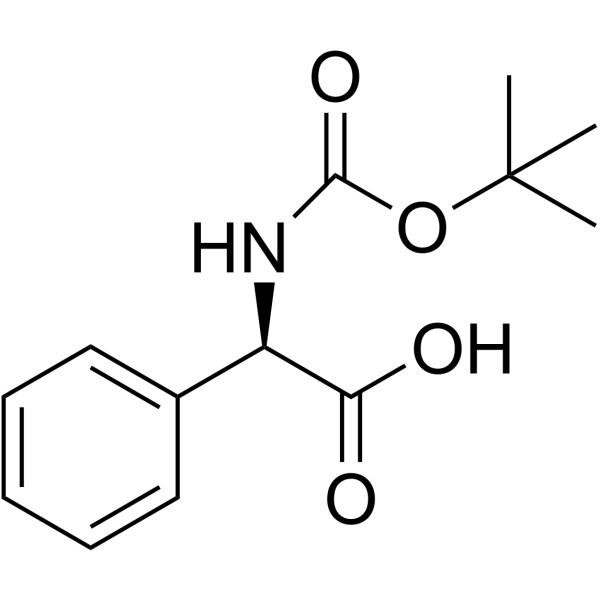
Cefaloglycin
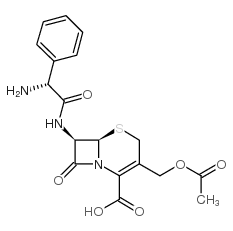
Cefaloglycin structure
|
Common Name | Cefaloglycin | ||
|---|---|---|---|---|
| CAS Number | 3577-01-3 | Molecular Weight | 405.42500 | |
| Density | 1.52 g/cm3 | Boiling Point | 761.8ºC at 760mmHg | |
| Molecular Formula | C18H19N3O6S | Melting Point | 236.5°C (rough estimate) | |
| MSDS | N/A | Flash Point | 414.5ºC | |
Use of CefaloglycinCefaloglycin (Cephaloglycin) is an orally active nephrotoxic β-lactam cephalosporin antibiotic with antibacterial activity. Cefaloglycin is activity against Gram-Positive cocci other than enterococci. Cefaloglycin is toxic to mitochondrial substrate uptake and respiration[1][2][3]. |
| Name | cefaloglycin |
|---|---|
| Synonym | More Synonyms |
| Description | Cefaloglycin (Cephaloglycin) is an orally active nephrotoxic β-lactam cephalosporin antibiotic with antibacterial activity. Cefaloglycin is activity against Gram-Positive cocci other than enterococci. Cefaloglycin is toxic to mitochondrial substrate uptake and respiration[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Gram-Positive cocci[2] |
| In Vitro | Cefaclor to be comparable to Cephaloglycin in terms of in vitro activity against S. pyogenes and somewhat superior to Cephalexin[2]. |
| In Vivo | Respiration with and the uptake of succinate and ADP are measured in rabbit renal cortical mitochondria exposed for 2 to 6 h to 300 to 3,000 µg of Cefaloglycin (Cephaloglycin) per mL and then washed to remove the antibiotic. Cefaloglycin (Cephaloglycin) irreversibly reduces both respiration and succinate uptake. The pattern of in vitro injury of Cefaloglycin to mitochondrial substrate uptake and respiration evolves in a time-dependent and concentration-dependent manner[1]. |
| References |
| Density | 1.52 g/cm3 |
|---|---|
| Boiling Point | 761.8ºC at 760mmHg |
| Melting Point | 236.5°C (rough estimate) |
| Molecular Formula | C18H19N3O6S |
| Molecular Weight | 405.42500 |
| Flash Point | 414.5ºC |
| Exact Mass | 405.09900 |
| PSA | 164.33000 |
| LogP | 1.01720 |
| Index of Refraction | 1.678 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
Cefaloglycin CAS#:3577-01-3 |
| Literature: Ogasa; Saito; Hashimoto; Sato; Hirata Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 2 p. 315 - 321 |
|
~% 
Cefaloglycin CAS#:3577-01-3 |
| Literature: Spencer; Flynn; Roeske; Siu; Chauvette Journal of medicinal chemistry, 1966 , vol. 9, # 5 p. 746 - 750 |
|
~% 
Cefaloglycin CAS#:3577-01-3 |
| Literature: Spencer; Flynn; Roeske; Siu; Chauvette Journal of medicinal chemistry, 1966 , vol. 9, # 5 p. 746 - 750 |
|
~% 
Cefaloglycin CAS#:3577-01-3 |
| Literature: Kawamori; Hashimoto; Katsumata; et al. Agricultural and Biological Chemistry, 1983 , vol. 47, # 11 p. 2503 - 2509 |
| Cefaloglycin |
| Cephaloglycine |
| (6R)-3-acetoxymethyl-7t-((R)-2-amino-2-phenyl-acetylamino)-8-oxo-(6rH)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cephaoglycin acid |
| (6R,7R)-3-(acetyloxymethyl)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cefaloglycine |
| Cefaloglicina |
| CEPHALOGLYCIN |
| D-Cephaloglycine |
| Kafocin |
| Cefaloglycinum |
| Cephaloglycin anhydrous |
![(6R)-3-acetoxymethyl-7t-((R)-2-tert-butoxycarbonylamino-2-phenyl-acetylamino)-8-oxo-(6rH)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid structure](https://image.chemsrc.com/caspic/219/7765-60-8.png)