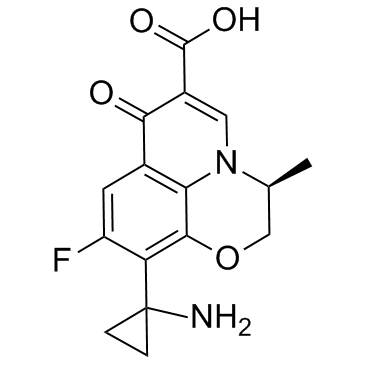
127045-41-4
| 中文名 | 帕珠沙星 |
|---|---|
| 英文名 | Pazufloxacin |
| 中文别名 |
(S)-(-)-10-(1-氨基环丙基)-9-氟-3-甲基-7-氧代-2,3-二氢-7H-吡啶并[1,2,3-DE][1,4]苯并恶嗪-6-羧酸甲磺酸盐
帕苏沙星 |
| 英文别名 |
T-3761
PAZUFLOXAXIN MFCD00865012 (-)-(3S)-10-(1-Aminocyclopropyl)-9-fluoro-2,3-dihydro-3-methyl-7--oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid T666 1A M BN EO KVT&&J C1 HF LVQ G- AL3TJ AZ &&S Form (3S)-10-(1-Aminocyclopropyl)-9-fluoro-3-methyl-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid ALKALYL (S)-10-(1-Aminocyclopropyl)-9-fluoro-2,3-dihydro-3-methyl-7-oxo-7h-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid Pazufloxaxin mesilate Pazufloxacin318.3 Pazufloxacin |
| 描述 | Pazufloxacin (T-3761)是氟喹诺酮类广谱抗生素。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 沸点 | 531.5±50.0 °C at 760 mmHg |
| 熔点 | 269-271°C |
| 分子式 | C16H15FN2O4 |
| 分子量 | 318.300 |
| 闪点 | 275.2±30.1 °C |
| 精确质量 | 318.101593 |
| PSA | 94.55000 |
| LogP | 0.09 |
| 外观性状 | 白色至淡,黄色结晶 |
| 蒸汽压 | 0.0±1.5 mmHg at 25°C |
| 折射率 | 1.692 |
| 储存条件 | 2-8°C |
| 稳定性 | 盐酸帕楚沙星(Pazufloxacin Hydrochloride):C16H15FN2O4?HCl。熔点243.5-247.5℃。[α]D23-32.5°(C=0.5,水)。 |
| 分子结构 | 1、 摩尔折射率:77.70 2、 摩尔体积(cm3/mol):202.8 3、 等张比容(90.2K):599.7 4、 表面张力(dyne/cm):76.4 5、 极化率(10-24cm3):30.80 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):-0.8 2.氢键供体数量:2 3.氢键受体数量:7 4.可旋转化学键数量:2 5.互变异构体数量:无 6.拓扑分子极性表面积92.9 7.重原子数量:23 8.表面电荷:0 9.复杂度:603 10.同位素原子数量:0 11.确定原子立构中心数量:1 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:棱状无色结晶。 2.熔点:259-259℃。 3.比旋光度:[α]D25-88.0°(C=0.5,0.05mol/L氢氧化钠溶液)。 |
|
毒理学数据: 急性毒性LD50雄小鼠(mg/kg):>500静脉注射。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H302-H312-H332 |
| 警示性声明 | P280 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) | Xn: Harmful; |
| 风险声明 (欧洲) | R20/21/22 |
| 安全声明 (欧洲) | S36/37 |
| 危险品运输编码 | NONH for all modes of transport |
| RTECS号 | UU8815300 |