Differences in the active site environment of Aspergillus ficuum phytases.
A H Ullah, K Sethumadhavan
文献索引:Biochem. Biophys. Res. Commun. 243(2) , 458-62, (1998)
全文:HTML全文
摘要
While Aspergillus ficuum phytaseA (phyA) was rapidly inactivated by 1,2-cyclohexanedione and phenylglyoxal, both specific modifiers of arginine, phytaseB (phyB) showed a markedly different behavior. First, phyB was totally insensitive to 1,2-cyclohexanedione even in the presence of 0.2 M guanidinium hydrochloride; second, the enzyme showed a great deal of resistance to inactivation by phenylglyoxal. Taken together, these results indicate that the chemical environment of the active site of phyB is very different from that of the active site of phyA. Despite sequence similarities of the active site region in these two proteins, their differential behavior to arginine modifiers indicates that other parts of the protein play a role in the active site formation. We expected some differences in the structure since the proteins have dissimilar kinetic parameters and pH optima.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
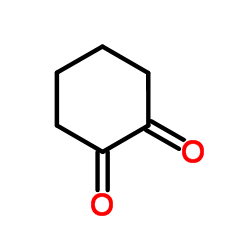 |
1,2-环己二酮
CAS:765-87-7 |
C6H8O2 |
|
Chemical modification of cationic groups in the polypeptide ...
1995-02-01 [Toxicon 33(2) , 187-99, (1995)] |
|
Modification of arginine-198 in sarcoplasmic reticulum Ca2+-...
1997-08-22 [J. Biol. Chem. 272(34) , 21142-50, (1997)] |
|
Evidence for an essential arginine in the flavoprotein nitro...
2001-01-01 [J. Enzym. Inhib. 16(2) , 157-63, (2001)] |
|
Modification of arginine residues in ovine prolactin by 1,2-...
1994-07-01 [Int. J. Pept. Protein Res. 44(1) , 31-5, (1994)] |
|
Identification of arginyl residues located at the ATP bindin...
1996-11-15 [J. Biol. Chem. 271(46) , 28933-41, (1996)] |