| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
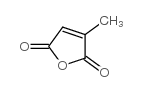 |
柠康酸酐
CAS:616-02-4 |
|
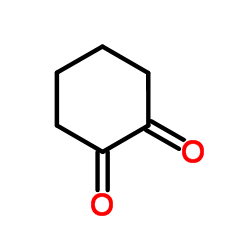 |
1,2-环己二酮
CAS:765-87-7 |
|
 |
Anthopleurin-A
CAS:60880-63-9 |