Evidence for an essential arginine in the flavoprotein nitroalkane oxidase.
G Gadda, A Banerjee, G S Fleming, P F Fitzpatrick
文献索引:J. Enzym. Inhib. 16(2) , 157-63, (2001)
全文:HTML全文
摘要
The flavoprotein nitroalkane oxidase from the fungus Fusarium oxysporum catalyzes the oxidative denitrification of primary or secondary nitroalkanes to yield the respective aldehydes or ketones, hydrogen peroxide and nitrite. The enzyme is inactivated in a time-dependent fashion upon treatment with the arginine-directed reagents phenylglyoxal, 2,3-butanedione, and cyclohexanedione. The inactivation shows first order kinetics with all reagents. Valerate, a competitive inhibitor of the enzyme, fully protects the enzyme from inactivation, indicating that modification is active site directed. The most rapid inactivation is seen with phenylglyoxal, with a k(inact) of 14.3 +/- 1.1 M(-1) min(-1) in phosphate buffer at pH 7.3 and 30 degrees C. The lack of increase in the enzymatic activity of the phenylglyoxal-inactivated enzyme after removing the unreacted reagent by gel filtration is consistent with inactivation being due to covalent modification of the enzyme. A possible role for an active site arginine in substrate binding is discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
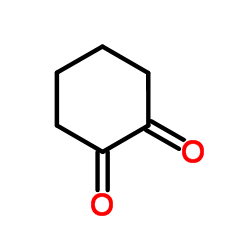 |
1,2-环己二酮
CAS:765-87-7 |
C6H8O2 |
|
Chemical modification of cationic groups in the polypeptide ...
1995-02-01 [Toxicon 33(2) , 187-99, (1995)] |
|
Modification of arginine-198 in sarcoplasmic reticulum Ca2+-...
1997-08-22 [J. Biol. Chem. 272(34) , 21142-50, (1997)] |
|
Modification of arginine residues in ovine prolactin by 1,2-...
1994-07-01 [Int. J. Pept. Protein Res. 44(1) , 31-5, (1994)] |
|
Identification of arginyl residues located at the ATP bindin...
1996-11-15 [J. Biol. Chem. 271(46) , 28933-41, (1996)] |
|
Albumin reduces basement membrane hydraulic conductance in p...
1995-11-01 [Am. J. Physiol. 269(5 Pt 2) , H1514-21, (1995)] |