| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
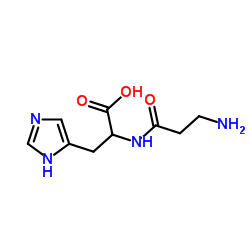 |
无花果蛋白酶,来源于无花果树乳胶
CAS:9001-33-6 |
|
 |
菠萝蛋白酶
CAS:37189-34-7 |
|
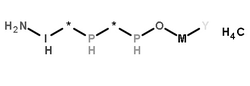 |
糜木瓜酶
CAS:9001-09-6 |