| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
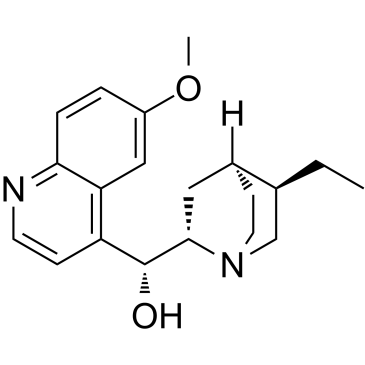 |
氢化奎宁
CAS:522-66-7 |
|
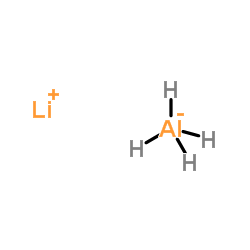 |
氢化铝锂
CAS:16853-85-3 |
|
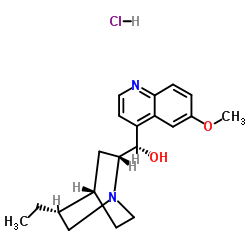 |
双氢奎尼丁盐酸盐
CAS:1476-98-8 |
|
 |
奎尼丁
CAS:56-54-2 |
|
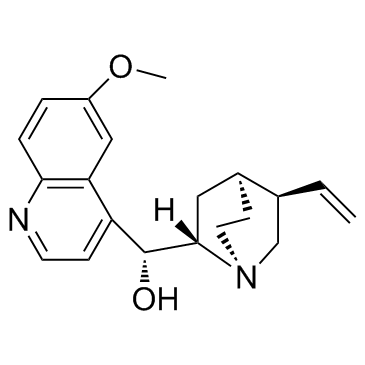 |
奎宁; 无水奎宁; 金鸡纳碱; 金鸡纳霜
CAS:130-95-0 |