Synthesis and alpha4beta2 nicotinic affinity of 2-pyrrolidinylmethoxyimines and prolinal oxime ethers.
Marco Pallavicini, Barbara Moroni, Cristiano Bolchi, Francesco Clementi, Laura Fumagalli, Cecilia Gotti, Silvia Vailati, Ermanno Valoti, Luigi Villa
文献索引:Bioorg. Med. Chem. Lett. 14(23) , 5827-30, (2004)
全文:HTML全文
摘要
Homochiral E and Z isomers of N-methylprolinal O-isopropyloxime and (1-methyl-2-pyrrolidinyl)methoxyimines were synthesized as candidate bioisosteres of nicotine and its isoxazolic analogue ABT 418. Two of them, namely (S)-2-isopropylideneaminooxymethyl- and (Z)-(S)-2-ethylideneaminooxymethyl-1-methylpyrrolidine, proved to bind at alpha4beta2 nicotinic acetylcholine receptor with submicromolar affinity and remarkable selectivity over alpha7 and muscarinic receptors thus supporting the hypothesized bioisosteric relationship between their methyloxyimino group and the aromatic heterocycles of the reference ligands.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
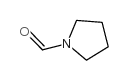 |
1-甲酰吡咯烷
CAS:3760-54-1 |
C5H9NO |
|
N-tritylprolinal: an efficient building block for the stereo...
2003-12-12 [J. Org. Chem. 68(25) , 9747-52, (2003)] |
|
Synthesis and inhibitory activity of acyl-peptidyl-prolinald...
1990-01-01 [J. Enzym. Inhib. 3(3) , 163-78, (1990)] |
|
Tyrosine hydroxylase deficiency in three Greek patients with...
2010-06-15 [Mov. Disord 25(8) , 1086-90, (2010)] |
|
New potent prolyl endopeptidase inhibitors: synthesis and st...
1994-06-24 [J. Med. Chem. 37(13) , 2071-8, (1994)] |
|
Mechanistic implications of the inhibition of peptidases by ...
1985-07-01 [Biochim. Biophys. Acta 829(3) , 311-8, (1985)] |