Mechanistic implications of the inhibition of peptidases by amino aldehydes and bestatin.
L Frick, R Wolfenden
文献索引:Biochim. Biophys. Acta 829(3) , 311-8, (1985)
全文:HTML全文
摘要
alpha-Amino aldehydes and bestatin are found to be effective inhibitors of a cytosolic dipeptidase (rat testicular peptidase C), and a cytosolic tripeptidase (rat kidney peptidase B, EC 3.4.11.4), as well as cytosolic leucine aminopeptidase (pig kidney peptidase S, EC 3.4.11.1). Aldehyde hydrates and bestatin share a resemblance to intermediates that might be formed during direct attack by water on peptide substrates, affording a possible explanation for their tight binding. Alternatively, inhibitors of both kinds might form derivatives of an active site nucleophile, resembling intermediates in a double-displacement mechanism. Exchange experiments with H218O suggest that bestatin is bound intact by leucine aminopeptidase, lending support to the first of these two mechanisms.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
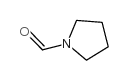 |
1-甲酰吡咯烷
CAS:3760-54-1 |
C5H9NO |
|
N-tritylprolinal: an efficient building block for the stereo...
2003-12-12 [J. Org. Chem. 68(25) , 9747-52, (2003)] |
|
Synthesis and inhibitory activity of acyl-peptidyl-prolinald...
1990-01-01 [J. Enzym. Inhib. 3(3) , 163-78, (1990)] |
|
Tyrosine hydroxylase deficiency in three Greek patients with...
2010-06-15 [Mov. Disord 25(8) , 1086-90, (2010)] |
|
New potent prolyl endopeptidase inhibitors: synthesis and st...
1994-06-24 [J. Med. Chem. 37(13) , 2071-8, (1994)] |
|
Secreted trypanosome cyclophilin inactivates lytic insect de...
2013-03-22 [J. Biol. Chem. 288(12) , 8772-84, (2013)] |