New potent prolyl endopeptidase inhibitors: synthesis and structure-activity relationships of indan and tetralin derivatives and their analogues.
Y Tanaka, S Niwa, H Nishioka, T Yamanaka, M Torizuka, K Yoshinaga, N Kobayashi, Y Ikeda, H Arai
文献索引:J. Med. Chem. 37(13) , 2071-8, (1994)
全文:HTML全文
摘要
New compounds were synthesized by structural modification of 1-[1-(4-phenylbutanoyl)-L-prolyl]-pyrrolidine (SUAM-1221, 1) or 1-[1-(benzyloxycarbonyl)-L-proly]prolinal (Z-Pro-prolinal,2) and were tested for in vitro inhibitory activities against purified prolyl endopeptidase (PEP) from canine brain. In a series of compounds which lack a formyl or a cyano group, 3-[3-[(S)-2-(1,2,3,4-tetrahydronaphthyl)acetyl]-L- thioprolyl]thiazolidine (13) exhibited an approximately 20-fold (IC50 = 2.3 nm) increase in potency compared with 1. Compounds having a formyl or a cyano group showed much more potent inhibitory activities than those which lack such a functional group. Among all compounds tested in vitro, 1-[1-(2-indanylacetyl)-L-prolyl]prolinal (27), 1-[1-[(S)-2-(1,2,3,4-tetrahydronaphthyl)acetyl]-L- prolyl]prolinal (29), 1-[3-[(S)-2-(1,2,3,4-tetrahydronaphthyl)-acetyl]-L-thioprolyl]p rolinal (30), (S)-2-cyano-1-[2-[(S)-2-(1,2,3,4-tetrahydronaphthyl)acetyl]-L- prolyl]pyrrolidine (34), and (S)-2-cyano-1-[3-[(S)-2-(1,2,3,4-tetrahydronaphthyl)acetyl]-L- thioprolyl]pyrrolidine (35) showed an approximately 2-fold (IC50 congruent to 0.5 nM) increase in potency compared with 2. The structure-activity relationships of these compounds are discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
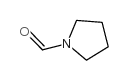 |
1-甲酰吡咯烷
CAS:3760-54-1 |
C5H9NO |
|
N-tritylprolinal: an efficient building block for the stereo...
2003-12-12 [J. Org. Chem. 68(25) , 9747-52, (2003)] |
|
Synthesis and inhibitory activity of acyl-peptidyl-prolinald...
1990-01-01 [J. Enzym. Inhib. 3(3) , 163-78, (1990)] |
|
Tyrosine hydroxylase deficiency in three Greek patients with...
2010-06-15 [Mov. Disord 25(8) , 1086-90, (2010)] |
|
Mechanistic implications of the inhibition of peptidases by ...
1985-07-01 [Biochim. Biophys. Acta 829(3) , 311-8, (1985)] |
|
Secreted trypanosome cyclophilin inactivates lytic insect de...
2013-03-22 [J. Biol. Chem. 288(12) , 8772-84, (2013)] |