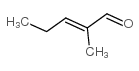
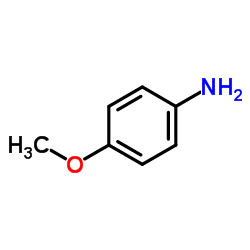
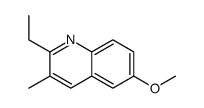
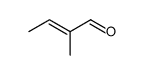
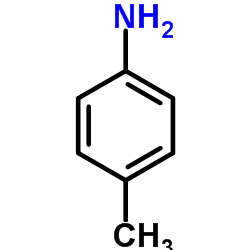
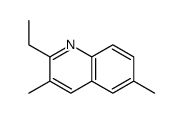
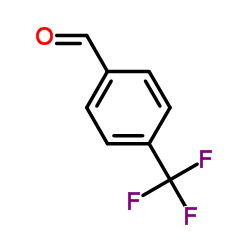
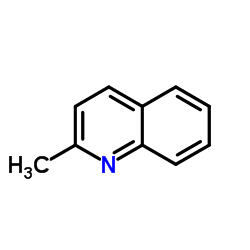
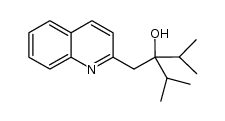
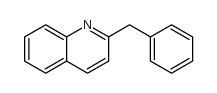
|
~32% |
|
~43% |
|
~27% |
|
~29% |
|
~% |
|
~70% |