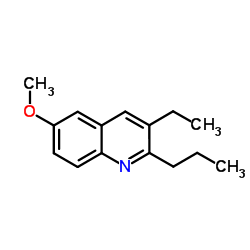
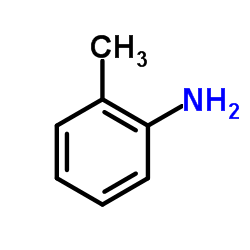
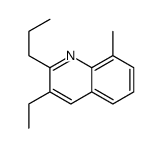
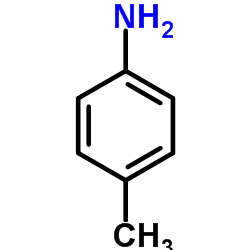
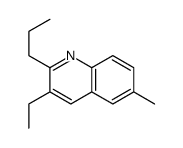
|
~78% |
|
~81% |
|
~63% |
|
~80% |
|
~67% |
|
~77% |
|
~72% |
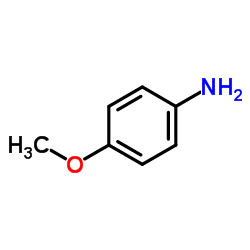

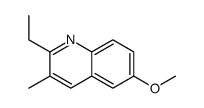
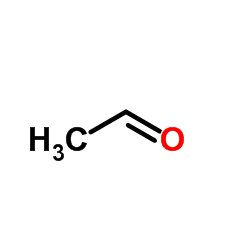
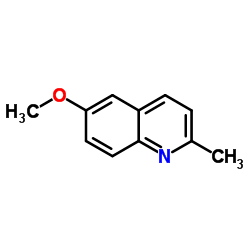
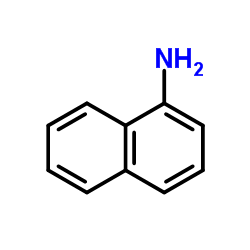
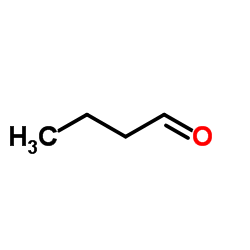
![3-ethyl-2-propylbenzo[h]quinoline结构式](https://image.chemsrc.com/caspic/102/61077-86-9.png)